Aedes aegypti - Factsheet for experts
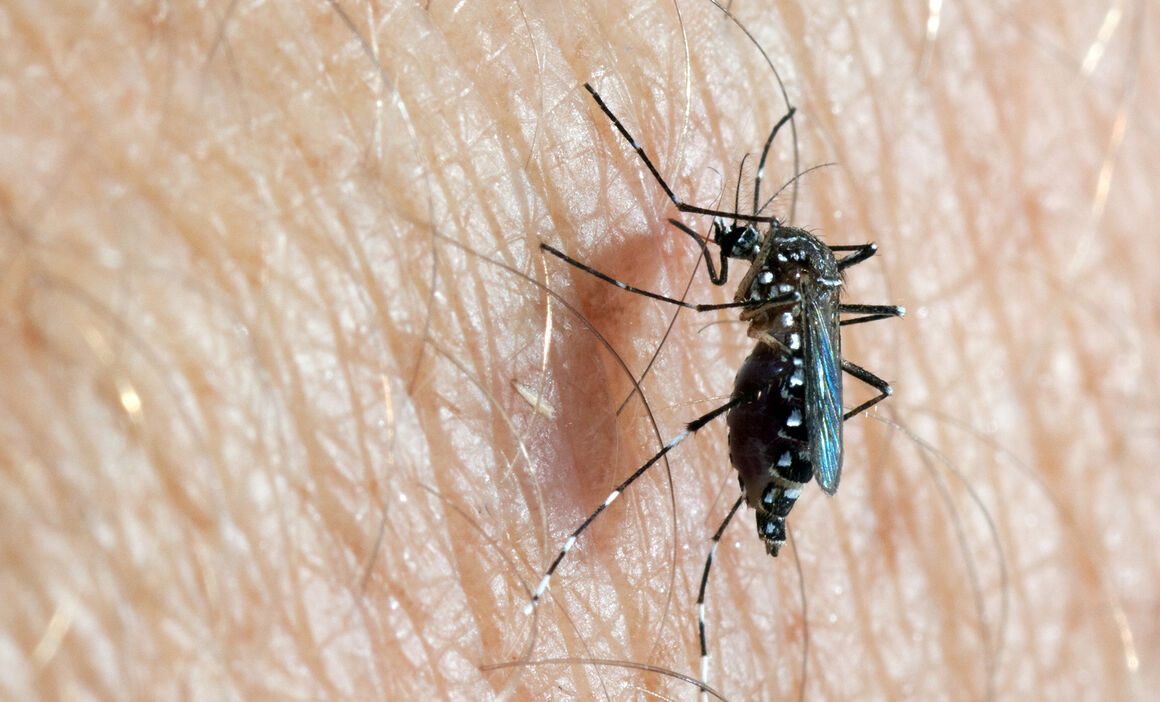
Species name/classification: Aedes (Stegomyia) aegypti
Common name: Yellow fever mosquito
Synonyms and other name in use: Stegomyia aegypti
Aedes aegypti is a known vector of several viruses including yellow fever virus, dengue virus, chikungunya virus, and Zika virus.
Index:
Hazards associated with mosquito species
Epidemiology and transmission of pathogens
Public health (control/interventions)
Hazards associated with mosquito species
Current issues
Invasive species
The invasive success of Ae. aegypti has largely been due to international travel and trade. Historically, Ae. aegypti has moved from continent to continent via ships and was previously established in southern Europe from the late 18th to the mid-20th century. Its disappearance from the Mediterranean, Black Sea and Macaronesian biogeographical region (Canary Islands, Madeira and the Azores) is not well understood [1,2]. It has since recolonised Madeira [3], reappeared in parts of southern Russia and Georgia (Krasnodar Krai and Abkhazia) [4], and reportedly been introduced into the Netherlands [5], Canary Islands [6,7] and Cyprus [8]. VectorNet field studies have shown the species to be widespread across extended areas of Georgia, including the capital city, Tbilisi, and it has also spread into north-eastern Türkiye [9]. Nowadays it is one of the most widespread mosquito species globally. If Ae. aegypti is introduced into southern Europe, there are no climatic or environmental reasons as to why it could not survive [10,11]. Dispersal via shipping (ferries) from Madeira is still thought to represent the greatest risk for the introduction of this mosquito into Europe. Although its global establishment is currently restricted due to its intolerance to temperate winters [13], over the past 30 years there has been an increase in its distribution worldwide [14].
Ecological plasticity
Ae. aegypti thrives in densely populated areas without reliable water supplies, waste management and sanitation [15]. It is suggested that Ae. aegypti evolved its domestic behaviour in West Africa, and its widespread colonisation and distribution across the tropics led to highly efficient inter-human transmission of viruses, such as dengue [16]. This domestic behaviour can provide protection from adverse environmental conditions (as it rests indoors) and offer numerous habitats suitable as oviposition sites, but also makes it vulnerable to (indoor) vector control measures [14].
Biting and disease risk
Aedes aegypti is a known vector of several viruses including yellow fever virus, dengue virus chikungunya virus and Zika virus. In Europe, imported cases infected with these viruses are reported every year [17,18]. Therefore, the potential establishment of this mosquito in Europe raises concerns about autochthonous transmission of these arboviruses [1-3,9,12,19], particularly in southern Europe where climatic conditions are most suitable for the re-establishment of the species. In 2012, a large outbreak of dengue fever, associated with Ae. aegypti, occurred in the Portuguese Autonomous Region of Madeira (on the African tectonic plate) [20]. The epidemic started in October 2012 and by early January 2013 more than 2 200 cases of dengue fever had been reported, with an additional 78 cases reported among European travellers returning from the island [21].
Geographical distribution
Historically Ae. aegypti has been reported as established in:
- all Mediterranean countries (Europe, Middle East and North Africa)
- in the Caucasus (southern Russia, Georgia, Azerbaijan)
- continental Portugal
- both the Atlantic archipelagos (Canaries and Azores) [2,22].
It is currently distributed throughout the tropics, including Africa (from where it originates) and a number of sub-tropical regions such as:
- south-eastern United States
- the Middle East
- South-East Asia
- the Pacific and Indian Islands
- northern Australia [23].
Although historically present in Europe, its current distribution is limited, but extending. The current known distribution of Ae. aegypti in Europe is displayed on the vector maps.
Brief history of spread and European distribution
Pathways
Aedes aegypti was most probably transported into the Americas and the Mediterranean on ships sailing from Africa [1,16,24]. The northernmost documented occurrences in Europe (Bordeaux and Saint Nazaire, France; Swansea and Southampton, UK) clearly result from introductions via ships, and there is no evidence that the species has become established in these places [2]. In the past, the species has been sporadically reported in Europe, from the Portuguese Atlantic coast to the Black Sea [2], displaying a much larger distribution than at present. The same also applies to North America and Australia [14]. The reduction in distribution is possibly due to elimination programmes.
Initial importations and spread in Europe
Aedes aegypti disappeared from Europe during the first half of the twentieth century (the species was reported in Spain up to 1953 and in Portugal up to 1956). Despite a few subsequent sporadic recordings (northern Italy, 1972; Israel, 1974; Turkey, 1961, 1984, 1992, 1993, 2001), it is only more recently that reports of re-colonisation have come to light [2]. Colonisation on the island of Madeira was reported as having started in 2004, and there are concerns that Aedes aegypti could be transported to western Europe via air or sea traffic [3]. Similarly, there are concerns that the species could be introduced into other countries bordering the Black Sea from Russia and Georgia via sea or road traffic, as this has already been shown to be the case in north-eastern Türkiye [9]. From there, the species could easily spread via road traffic to other parts of Türkiye, including Istanbul, and on to neighbouring EU states. Furthermore, Ae. aegypti has been reported to have been found in the Netherlands at tyre yards, undoubtedly imported via shipments of tyres originating from Florida, USA [5,25]. However, the control measures that were immediately applied have successfully eliminated the species from these foci.
Possible future expansion
Unlike Ae. albopictus, the ability of Ae. aegypti to establish itself in more temperate regions is currently restricted, due to its intolerance of temperate winters and, in particular, the high mortality rate of eggs when exposed to frost [13,26]. However, there is no reason why it should not become re-established widely across the Mediterranean. Coastal regions of the Mediterranean, the Black Sea, and the Caspian Sea, and areas along large lowland rivers (Ebro, Garonne, Rhone, and Po) have been identified as suitable habitats for Ae. aegypti [10]. Moreover, this could change in the future, with global climate change resulting in the species’ ability to expand further to the north and south [16]. Back to Top
Entomology
Species name/classification: Aedes (Stegomyia) aegypti (Linnaeus, 1762) [27]
Common name: Yellow fever mosquito
Synonyms and other name in use: Stegomyia aegypti (sensu Reinert et al., 2004) [28]
Morphological characters and similar species
Adults of Ae. aegypti are relatively small and have a black and white pattern due to the presence of white/silver scale patches against a black background on the legs and other parts of the body. Some indigenous mosquitoes also show such contrasts (more brownish and yellowish) but these are less obvious. However, Ae. Aegypti could be confused with other invasive (Ae. Albopictus, Ae. Japonicus) or indigenous species (Ae. Cretinus, restricted to Cyprus, Greece and Türkiye). The prevailing diagnostic character is the presence of silver scales in the shape of a lyre against a black background on the scutum (dorsal part of the thorax). The domestic form (Ae. Aegypti aegypti) is paler than its ancestor (Ae. Aegypti formosus) and has white scales on the first abdominal tergite. The latter is confined to Africa, south of the Sahara, and has been recorded as breeding in natural habitats in areas of forest or bush, away from places of human settlement [29].
Seasonal abundance
On the island of Madeira Ae. aegypti is active throughout the year, with a peak in abundance from August to October [30].
Voltinism (generations per season)
Multivoltine
Host preferences (e.g. birds, mammals, humans)
Aedes aegypti feeds on mammalian hosts [31], preferably humans, even in the presence of alternative hosts [32]. It also feeds multiple times during one gonotrophic cycle (feeding, egg-producing cycle) [14,16,33] which has implications for disease transmission.
Aquatic/terrestrial habitats
Historically, Ae. aegypti was found in forested areas, using tree holes as habitats [16]. As an adaptation to urban domestic habitats, nowadays it exploits a wide range of artificial containers such as vases, water tanks and tyres [14]. It also uses underground aquatic habitats, such as septic tanks [34], and can adapt to use both indoor and outdoor aquatic container habitats in the same area. Adaptation to breeding outdoors may result in increased population numbers and difficulty in implementing control methods [32]. A study in Brazil found high numbers of eggs in oviposition sites close to human populations [35]. Eggs which are laid on or near the water surface [14] are normally resistant to desiccation [36].
Biting/resting habits
The domestic form of Ae. aegypti is often found as close as 100 metres to human habitations [1] although some studies have shown that breeding habitats can also be found away from human dwellings [32]. Aedes aegypti prefer human habitations as they provide resting and host-seeking possibilities [16] and, as a result, they will readily enter buildings [1,14]. The activity of the species is both diurnal and crepuscular [14,31].
Environmental thresholds/constraints/development criteria
Aedes aegypti, unlike Ae. albopictus, is not able to undergo winter diapause as eggs, and this therefore limits its ability to exploit more northerly temperate regions (although some survival is possible during the summer following an importation). However, it may establish itself in regions of Europe with a humid sub-tropical climate (e.g. parts of the Mediterranean and countries around the Black Sea), such as the Sochi region where it has become re-established since 2001 [37]. Species competition has also been shown to affect distribution and abundance. A decrease in the distribution of Ae. aegypti has been associated with the invasion of Ae. albopictus, especially in south-eastern USA [14].
Aedes aegypti also has limited dispersal capability in its adult form [14], with a flight range estimated to be only 200 metres [31]. Rainfall may affect abundance and productivity of breeding sites but this species’ preference for artificial water containers means it does not have to rely on rainfall for the availability of larval development sites [14]. These aspects, coupled with its preference for feeding and resting indoors, make the species less susceptible to the effects of climatic factors, which could influence its distribution.
Epidemiology and transmission of pathogens
Known vector status
Aedes aegypti is known to transmit dengue virus, yellow fever virus, chikungunya virus, and Zika virus. It has been suggested as a potential vector of Venezuelan Equine Encephalitis virus [38] and vector competency* studies have shown that Ae. aegypti is capable of transmitting West Nile virus. West Nile virus has also been isolated from this mosquito species in the field [31].
Chikungunya
Aedes aegypti is the primary vector of chikungunya virus [39]. Transovarial transmission was demonstrated by Aitken et al. [40] under laboratory conditions and the virus has been detected in wild-caught male Ae. aegypti [41]. Transovarial transmission may help with the maintenance of the virus in nature [42]. Venereal transmission during mating has also been demonstrated under laboratory conditions, although it is thought to be less widespread than transovarial transmission [42].
Aedes aegypti has been involved in virtually all chikungunya epidemics in Africa, India and other countries in South-East Asia [42][43]. The species caused an outbreak of chikungunya in Kenya (2004) and the Comoros islands (2005), affecting 63% of the population in the latter case [44]. An entomological investigation following an outbreak of chikungunya virus in Yemen (2010/2011) revealed the presence of the virus in field-collected Ae. aegypti in the outbreak area [45]. More recently, Ae. aegypti was involved in large chikungunya outbreaks in the Pacific and the Caribbean [19,46,47]. As a consequence, Europe’s vulnerability to the virus has increased [17,19].
More information on the disease can be found on the fact sheet about chikungunya.
Dengue
Aedes aegypti is the primary vector of dengue [48]. All four dengue serotypes have been isolated from field-collected Ae. aegypti [49]. Vertical transmission of dengue virus types 2, 3 and 4 has been demonstrated [29] and although some suggest this is inefficient [49], others suggest that it plays a significant role in viral maintenance [50].
Aedes aegypti has long been recognised as a vector of dengue, causing major dengue fever epidemics in the Americas and South-East Asia. The global incidence of dengue has also increased over the past 25 years [14,51]. Historically, outbreaks have also been reported in Europe, with one of the largest outbreaks on record occurring in Athens and neighbouring areas of Greece during the period 1927–1928 [52] [2]. In 2012, a large outbreak of dengue fever occurred in the Portuguese Autonomous Region of Madeira [20] where Ae. aegypti is established.
More information on the disease can be found on the fact sheet about dengue.
Yellow fever
Yellow fever is maintained in a sylvatic cycle between monkeys and mosquitoes of Aedes or Haemagogus genera [29,53]. Aedes aegypti is the vector involved in urban transmission of yellow fever where only humans are the amplifying host. Aedes aegypti has been shown to transmit yellow fever virus transovarially to F1 progeny under laboratory conditions [40] and field collection studies have also confirmed this in nature [29].
Yellow fever transmission has been reported from countries across sub-Saharan Africa and in tropical areas across South and Central America, from Panama to the northern part of Argentina [54]. Autochthonous transmission of yellow fever has never been detected in Asia, although the Ae. aegypti vector is present in south and south-eastern areas of the continent [55].
More information on the disease can be found on the fact sheet about yellow fever.
Zika virus
Zika virus is maintained in a sylvatic cycle involving non-human primates and a wide variety of sylvatic and peri-domestic Aedes mosquitoes. Aedes aegypti is considered the most important vector for Zika virus transmission to humans. Aedes aegypti mosquitoes were found infected in the wild (reviewed in [56]). More recently, the species was found infected during the Zika virus outbreak in Brazil [57]. The mosquito has been shown to transmit the virus under laboratory conditions but differences in vector competence* between studies were reported [58-60].
More information on the disease can be found on the fact sheet Zika virus infection
Factors driving/impacting on transmission cycles
The spread of Ae. aegypti-borne diseases has been aided by the global spread of Ae. aegypti over the past 25 years [14]. Although currently limited in spread due to its intolerance to temperate winters, climate change could result in an increased distribution of Ae. aegypti.
As the human population grows, sites in which this mosquito can thrive will increase, providing further habitats. This fact, coupled with the close proximity of humans and the tendency of Ae. aegypti to feed on multiple hosts during one gonotrophic cycle [16][14][33], increases the risk of disease transmission in such areas. The movement of viraemic hosts can result in outbreaks from a number of arboviruses in non-endemic areas.
The re-establishment of Ae. aegypti in some areas has resulted in disease transmission. Inadequate control of this invasive species could lead to its re-establishment in Europe which is why surveillance and research on this mosquito is so important.
Public health (control/interventions)
Vector surveillance
Methods for surveying Ae. aegypti are addressed in ECDC’s ‘Guidelines for the surveillance of invasive mosquitoes in Europe’ [61].
ECDC and the European Food Safety Authority (EFSA) fund European-wide monitoring and mapping activities for invasive mosquito species and potential mosquito vectors (VectorNet).
Species specific control methods
Source reduction and adult control
Aedes aegypti thrives in urban environments which provide it with numerous oviposition sites to lay eggs. Therefore, the distribution of this species is largely driven by human activities (e.g. storage of water outside) and this should be the focus of control methods [14]. This is challenging because of the numerous sites in which Ae. aegypti lay eggs and in an urban setting, such sites are hard to access. For example, a study in Mexico used a combination of quadrat and transect sampling methods to identify the most important containers for pupal development in 600 houses. They found an association between Ae. aegypti pupae and large cement washbasins. Source reduction and targeted treatment of such sites could ensure that the use of insecticides is more successful in reducing mosquito numbers [62].
Historically, outbreaks of dengue and yellow fever have been controlled by Ae. aegypti eradication programmes but these have not always been successful and abandoning efforts led to the re-emergence of the diseases associated with this mosquito [63]. In the twentieth century, many eradication programmes were targeted at larval development sites in an attempt to eliminate yellow fever transmission. The use of DDT after the Second World War resulted in the eradication of the species from 22 countries in the Americas [1]. This effort was discontinued and Ae. aegypti quickly re-colonised nearly all of the neo-tropics and sub-tropics [16]. Since Ae. aegypti has become less accessible, due to the fact that the species spends more time indoors, outdoor insecticidal spraying has become less efficient [1]. Eradication programmes set up during the 1950–60s (initiated by the Pan American Health Organization) in the Americas saw the reduction and eradication of Ae. aegypti there, but relaxation of mosquito management after the 1970s resulted in the re-establishment of Ae. aegypti, followed by dengue outbreaks [14].
Some other methods used include the introduction of predators into the larval habitats of Ae. aegypti (e.g. copepods), the introduction of irradiated or genetically-modified mosquitoes (sterile male release) and the use of Wolbachia bacteria which can inhibit the replication of dengue virus within Ae. aegypti, thereby suppressing or eliminating dengue transmission [14]. Protective clothing and repellents are also advocated to reduce exposure to Ae. aegypti, as well as the spraying of indoor living spaces with pyrethrin [53]. Personal protective measures to reduce the risk of mosquito bites also include using mosquito bed nets (preferably insecticide-treated nets), and sleeping or resting in screened or air-conditioned rooms.
Integrated control programme
Implementation of an integrated control strategy against invasive mosquito species should take into account the target species, its ecology and the public health concern (i.e. nuisance and/or disease transmission). As a general rule, an integrated control strategy requires the coordinated involvement of local authorities, private partners, organised society and communities [64].
Traditional methods such as source reduction, public education and insecticide application are routinely implemented by municipalities to reduce Aedes populations, but with limited success, probably because of poor participation of communities, and a lack of coordination and synchronised implementation [64]. Innovative approaches, such as pyriproxyfen autodissemination and genetic or Wolbachia-based methods, still have to be developed to demonstrate their efficacy and sustainability, but could be considered in future integrated programmes.
It is suggested that mosquito control programmes should be more effective against Ae. aegypti (as opposed to Ae. albopictus) due to its strong urban presence and preference for feeding on humans [13]. Using a combination of control methods as opposed to one strategy is suggested to be most effective, and will reduce the chance of introducing selective pressures - e.g. on oviposition site selection [65]. However, following the discovery of Ae. aegypti in Madeira, using a combined control strategy of spraying insecticides, reducing potential breeding sites and increasing public health awareness did not prevent the species from re-establishing itself there [3].
Existing public health awareness and education materials
ECDC provides regularly-updated vector distribution maps and guidelines for the surveillance of invasive mosquitoes [61].
The US Centers for Disease Prevention and Control (US CDC) provide advice for travellers on protection against mosquitoes, ticks and other arthropods: http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/protection-against-mosquitoes-ticks-insects-arthropods.aspx.
Key areas of uncertainty
It is clear that if Ae. aegypti re-establishes itself in the European regions it previously inhabited and spreads, it will have a significant impact on public health. The spread of Ae. aegypti needs to be monitored as this species is the primary vector of dengue, chikungunya, yellow fever and Zika viruses.
Footnote
*Vector competence is the physiological ability of a mosquito to become infected with and transmit a pathogen, and is typically assessed in laboratory studies. In nature, transmission of a pathogen by vectors is dependent not only on vector competence but also on factors describing the intensity of interaction between the vector, the pathogen and the host in the local environment. Therefore, vector and host densities, geographic distribution, longevity, dispersal and feeding preferences have to be considered to determine the vectorial capacity of a vector population and its role in transmission
Read more
Reverse identification key for mosquito species
References
1. Reiter P. Yellow fever and dengue: a threat to Europe? Eurosurveillance. 2010 Mar 11;15(10):19509.
2. Schaffner F, Mathis A. Dengue and dengue vectors in the WHO European Region: past, present, and scenarios for the future. Lancet Infectious Diseases. 2014;14(12):1271-80.
3. Almeida AP, Goncalves YM, Novo MT, Sousa CA, Melim M, Gracio AJ. Vector monitoring of Aedes aegypti in the Autonomous Region of Madeira, Portugal. Eurosurveillance. 2007 Nov;12(11):E071115 6.
4. Yunicheva YU, Ryabova TE, Markovich NY, Bezzhonova OV, Ganushkina LA, Semenov VB, et al. First data on the presence of breeding populations of the Aedes aegypti L. mosquito in Greater Sochi and various cities of Abkhazia. Meditsinskaia Parazitologiia I Parazitarnye Bolezni 2008;3:40-3.
5. Scholte E, Den Hartog W, Dik M, Schoelitsz B, Brooks M, Schaffner F, et al. Introduction and control of three invasive mosquito species in the Netherlands, July-October 2010. Eurosurveillance. 2010;15(45):19710.
6. Barceló C, Blanda V, del Castillo-Remiro A, Chaskopoulou A, Connelly CR, Ferrero-Gómez L, et al. Surveillance of invasive mosquito species in islands with focus on potential vectors of zoonotic diseases. In: Gutiérrez-López R, Logan JG, Martínez-de la Puente J (editors). Ecology of diseases transmitted by mosquitoes to wildlife. Wageningen: Wageningen Academic Publishers; 2022. (p. 264).
7. Sanidad activa el Sistema de Vigilancia Entomológica ante la detección de ejemplares de Aedes aegypti en Tenerife: Fundacion Canaria para el control de las enfermedades tropicales; [updated 23/12/2022 and 18/01/2023]. Available from: https://funccet.es/mosquito-aedes-aegypti-tenerife/
8. Shkurko J. Action plan drawn up for yellow fever mosquito: CyprusMail; 2022. Available from: https://cyprus-mail.com/2022/10/05/action-plan-drawn-up-for-yellow-fever-mosquito/
9. Akiner MM, Demirci B, Babuadze G, Robert V, Schaffner F. Spread of the Invasive Mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea Region Increases Risk of Chikungunya, Dengue, and Zika Outbreaks in Europe. PLoS Neglected Tropical Diseases. 2016;10(4):e0004664.
10. European Centre for Disease Prevention and Control (ECDC). The climatic suitability for dengue transmission in continental Europe. Stockholm: ECDC; 2012. Available from: https://www.ecdc.europa.eu/en/publications-data/climatic-suitability-dengue-transmission-continental-europe
11. Wint W, Jones P, Kraemer M, Alexander N, Schaffner F. Past, present and future distribution of the yellow fever mosquito Aedes aegypti: The European paradox. Sci Total Environ. 2022 Nov 15;847:157566.
12. Rogers DJ, Suk JE, Semenza JC. Using global maps to predict the risk of dengue in Europe. Acta Trop. 2014 Jan;129:1-14.
13. Gould EA, Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg. 2009 Feb;103(2):109-21.
14. Jansen CC, Beebe NW. The dengue vector Aedes aegypti: what comes next? Microbes Infect. 2010 Apr;12(4):272-9.
15. Honorio NA, Codeco CT, Alves FC, Magalhaes MA, Lourenco-De-Oliveira R. Temporal distribution of Aedes aegypti in different districts of Rio de Janeiro, Brazil, measured by two types of traps. J Med Entomol. 2009 Sep;46(5):1001-14.
16. Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010 Feb;85(2):328-45.
17. Paty MC, Six C, Charlet F, Heuze G, Cochet A, Wiegandt A, et al. Large number of imported chikungunya cases in mainland France, 2014: a challenge for surveillance and response. Eurosurveillance. 2014;19(28):20856.
18. European Centre for Disease Prevention and Control (ECDC). Surveillance Atlas of Infectious Diseases Stockholm: European Centre for Disease Prevention; 2016. Available from: http://atlas.ecdc.europa.eu/public/index.aspx?Instance=GeneralAtlas
19. Van Bortel W, Dorleans F, Rosine J, Blateau A, Rousset D, Matheus S, et al. Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. 2014;19(13):20759.
20. Sousa CA, Clairouin M, Seixas G, Viveiros B, Novo MT, Silva AC, et al. Ongoing outbreak of dengue type 1 in the Autonomous Region of Madeira, Portugal: preliminary report. Eurosurveillance. 2012;17(49):20333.
21. European Centre for Disease Prevention and Control (ECDC). Epidemiological update: outbreak of dengue in Madeira, Portugal. Stockholm: ECDC; 2013. Available from: http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?List=8db7286c-fe2d-476c-9133-18ff4cb1b568&ID=23
22. Holstein M. Dynamics of Aedes aegypti distribution, density and seasonal prevalence in the Mediterranean area. Bull World Health Organ. 1967;36(4):541-3.
23. Soumahoro MK, Fontenille D, Turbelin C, Pelat C, Boyd A, Flahault A, et al. Imported chikungunya virus infection. Emerg Infect Dis. 2010 Jan;16(1):162-3.
24. Eritja R, Escosa R, Lucientes J, Marques E, Roiz D, Ruiz S. Worldwide invasion of vector mosquitoes: present European distribution and challenges in Spain. Biological Invasions 2005;7(1).
25. Brown JE, Scholte EJ, Dik M, Den Hartog W, Beeuwkes J, Powell JR. Aedes aegypti mosquitoes imported into the Netherlands, 2010. Emerg Infect Dis. 2011 Dec;17(12):2335-7.
26. Otero M, Solari HG, Schweigmann N. A stochastic population dynamics model for Aedes aegypti: formulation and application to a city with temperate climate. Bulletin of Mathematical Biology. 2006 Nov;68(8):1945-74.
27. Wilkerson RC, Linton YM, Fonseca DM, Schultz TR, Price DC, Strickman DA. Making Mosquito Taxonomy Useful: A Stable Classification of Tribe Aedini that Balances Utility with Current Knowledge of Evolutionary Relationships. PLoS One. 2015;10(7):e0133602.
28. Reinert JF, Harbach RE, Kitching IJ. Phylogeny and classification of Aedini (Diptera: Culicidae), based on morphological characters of all life stages. Zool J Linn Soc-Lond. 2004 Nov;142(3):289-368.
29. Fontenille D, Diallo M, Mondo M, Ndiaye M, Thonnon J. First evidence of natural vertical transmission of yellow fever virus in Aedes aegypti, its epidemic vector. Trans R Soc Trop Med Hyg. 1997 Sep-Oct;91(5):533-5.
30. Gonçalves Y, Silva J, Biscotto M. On the presence of Aedes (Stegomyia) aegypti Linnaeus, 1762 (Insecta, Diptera, Culicidae) in the island of Madeira (Portugal). Boletim do Museu Municipal do Funchal. 2008;58(322):53-9.
31. Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. An update on the potential of north American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005 Jan;42(1):57-62.
32. Saifur RG, Dieng H, Hassan AA, Salmah MR, Satho T, Miake F, et al. Changing domesticity of Aedes aegypti in northern peninsular Malaysia: reproductive consequences and potential epidemiological implications. PLoS One. 2012;7(2):e30919.
33. Scott TW, Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends in Parasitology. 2012 Mar;28(3):114-21.
34. Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol. 2008 Mar;22(1):62-9.
35. Medeiros AS, Marcondes CB, De Azevedo PR, Jeronimo SM, e Silva VP, Ximenes Mde F. Seasonal variation of potential flavivirus vectors in an urban biological reserve in north-eastern Brazil. J Med Entomol. 2009 Nov;46(6):1450-7.
36. Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005 May;8(5):558-74.
37. Ryabova TY, Yunicheva YV, Markovich NY, Ganushkina LA, Orabei VG, Sergiev VP. Detection of Aedes (Stegomyia) aegypti L. mosquitoes in Sochi. Meditsinskaia Parazitologiia i Parazitarnye Bolezni 2005;Jul-Sept:3-5.
38. Larsen JR, Ashley RF. Demonstration of Venezuelan equine encephalomyelitis virus in tissues of Aedes Aegypti. Am J Trop Med Hyg. 1971 Sep;20(5):754-60.
39. de Lamballerie X, Leroy E, Charrel RN, Ttsetsarkin K, Higgs S, Gould EA. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol J. 2008;5:33.
40. Aitken TH, Tesh RB, Beaty BJ, Rosen L. Transovarial transmission of yellow fever virus by mosquitoes (Aedes aegypti). Am J Trop Med Hyg. 1979 Jan;28(1):119-21.
41. Thavara U, Tawatsin A, Pengsakul T, Bhakdeenuan P, Chanama S, Anantapreecha S, et al. Outbreak of Chikungunya Fever in Thailand and Virus Detection in Field Population of Vector Mosquitoes, Aedes Aegypti (L.) and Aedes Albopictus Skuse (Diptera: Culicidae). Se Asian J Trop Med. 2009 Sep;40(5):951-62.
42. Mavale M, Parashar D, Sudeep A, Gokhale M, Ghodke Y, Geevarghese G, et al. Venereal transmission of chikungunya virus by Aedes aegypti mosquitoes (Diptera: Culicidae). Am J Trop Med Hyg. 2010 Dec;83(6):1242-4.
43. Vega-Rua A, Lourenco-de-Oliveira R, Mousson L, Vazeille M, Fuchs S, Yebakima A, et al. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Neglected Tropical Diseases. 2015 May;9(5):e0003780.
44. Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009 Sep 15;49(6):942-8.
45. Zayed A, Awash AA, Esmail MA, Al-Mohamadi HA, Al-Salwai M, Al-Jasari A, et al. Detection of Chikungunya virus in Aedes aegypti during 2011 outbreak in Al Hodayda, Yemen. Acta Trop. 2012 Jul;123(1):62-6.
46. Dupont-Rouzeyrol M, Caro V, Guillaumot L, Vazeille M, D'Ortenzio E, Thiberge JM, et al. Chikungunya virus and the mosquito vector Aedes aegypti in New Caledonia (South Pacific Region). Vector Borne and Zoonotic Diseases. 2012;12(12):1036-41.
47. Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, et al. Concurrent outbreaks of dengue, chikungunya and Zika virus infections - an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012-2014. Eurosurveillance. 2014;19(41):20929.
48. Ramchurn SK, Moheeput K, Goorah SS. An analysis of a short-lived outbreak of dengue fever in Mauritius. Eurosurveillance. 2009;14(34):19314.
49. Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004 Sep;18(3):215-27.
50. Mulyatno KC, Yamanaka A, Yotopranoto S, Konishi E. Vertical transmission of dengue virus in Aedes aegypti collected in Surabaya, Indonesia, during 2008-2011. Japanese Journal of Infectious Diseases. 2012;65(3):274-6.
51. Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infectious Diseases. 2016 Jun;16(6):712-23.
52. Rosen L. Dengue in Greece in 1927 and 1928 and the pathogenesis of dengue hemorrhagic fever: new data and a different conclusion. The American Journal of Tropical Medicine and Hygiene. 1986 May;35(3):642-53.
53. Monath TP, Cetron MS. Prevention of yellow fever in persons traveling to the tropics. Clin Infect Dis. 2002 May 15;34(10):1369-78.
54. World Health Organization (WHO). List of countries, territories and areas. Yellow fever vaccination requirements and recommendations; malaria situation; and other vaccination requirements. Geneva: WHO; 2015.
55. Agampodi SB, Wickramage K. Is there a risk of yellow fever virus transmission in South Asian countries with hyperendemic dengue? Biomed Res Int. 2013;2013:905043.
56. Musso D, Gubler DJ. Zika Virus. Clin Microbiol Rev. 2016 Jul;29(3):487-524.
57. Ferreira-de-Brito A, Ribeiro IP, Miranda RM, Fernandes RS, Campos SS, Silva KA, et al. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Memórias do Instituto Oswaldo Cruz. 2016 Oct;111(10):655-8.
58. Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. Plos Neglected Tropical Diseases. 2016 Mar;10(3):e0004543.
59. Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. Plos Neglected Tropical Diseases. 2012;6(8):e1792.
60. Diagne CT, Diallo D, Faye O, Ba Y, Faye O, Gaye A, et al. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infectious Diseases. 2015;15:492.
61. European Centre for Disease Prevention and Control (ECDC). Guidelines for the surveillance of invasive mosquitoes in Europe. Stockholm: ECDC; 2012. Available from: https://www.ecdc.europa.eu/en/publications-data/guidelines-surveillance-invasive-mosquitoes-europe
62. Arredondo-Jimenez JI, Valdez-Delgado KM. Aedes aegypti pupal/demographic surveys in southern Mexico: consistency and practicality. Ann Trop Med Parasitol. 2006 Apr;100 Suppl 1:S17-S32.
63. Gubler DJ. Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis. 1998 Jul-Sep;4(3):442-50.
64. Baldacchino F, Caputo B, Chandre F, Drago A, della Torre A, Montarsi F, et al. Control methods against invasive Aedes mosquitoes in Europe: a review. Pest Management Science. 2015 Nov;71(11):1471-85.
65. Wong J, Morrison AC, Stoddard ST, Astete H, Chu YY, Baseer I, et al. Linking oviposition site choice to offspring fitness in Aedes aegypti: consequences for targeted larval control of dengue vectors. PLoS Neglected Tropical Diseases. 2012;6(5):e1632.




