Elimination targets progress
Prevention measures: progress towards the elimination targets
A visual summary of the progress towards the European Action Plan 2020 for hepatitis elimination is shown in Figure 3, indicating the number of countries meeting the target, not meeting the target, or with no data reported. The charts reflect data from countries where the indicator is relevant because there is a corresponding policy or programme in place. Therefore, some charts do not have the same number of countries represented. Additionally, as there are no data available on the percentage of infections administered with safety-engineered injection devices, that target is not included in the figure. The figure highlights gaps in reported data for these targets, which may be due to data unavailability at the local level or due to challenges in the reporting of the data.
Progress towards the WHO European Action Plan Hepatitis Prevention 2020 Targets: at a glance
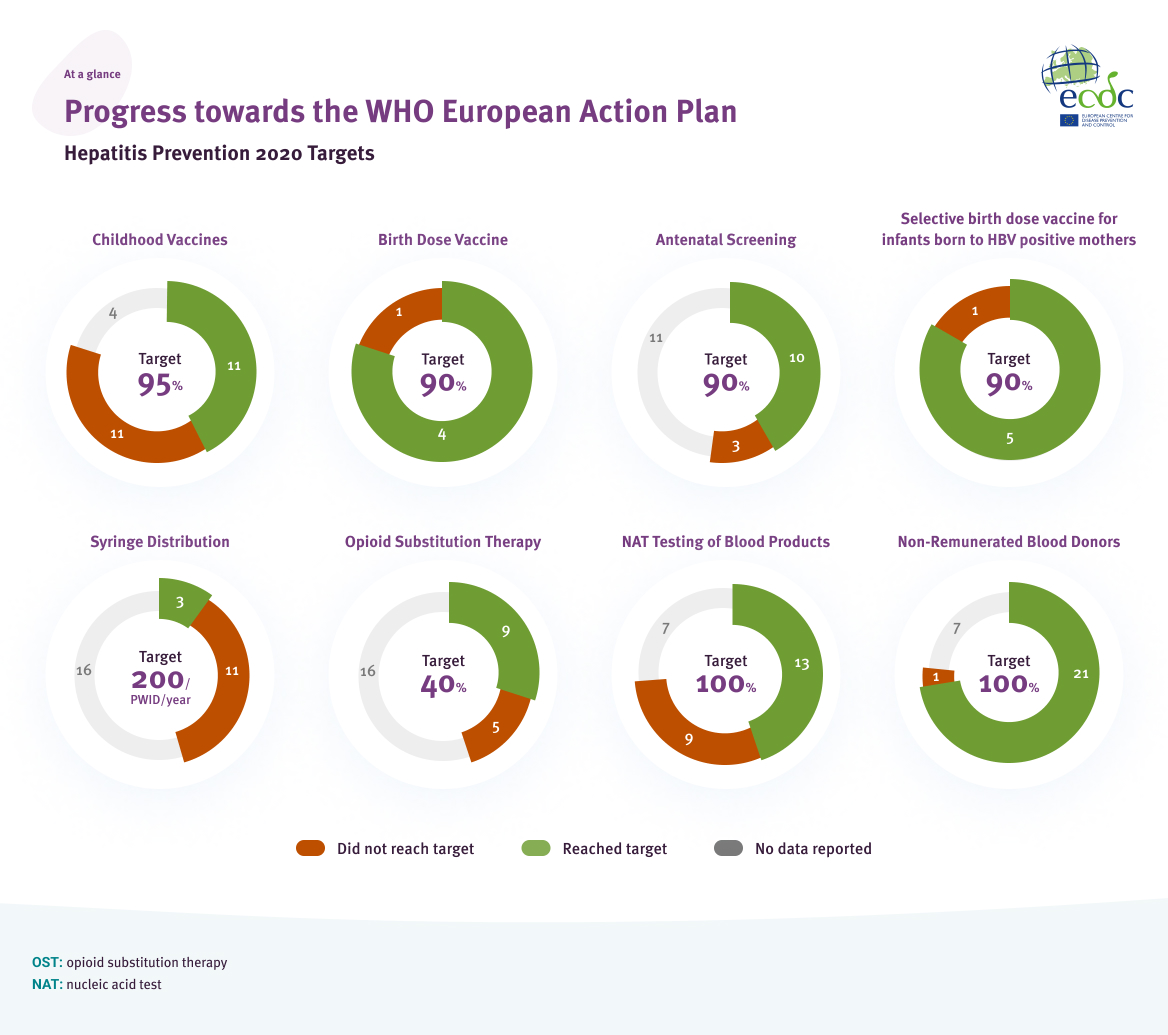
Source: Data reported from EU/EEA countries through ECDC hepatitis monitoring survey, 2021, and WHO/UNICEF coverage estimates, EDQM (Council of Europe). The collection, testing and use of blood and blood components in Europe: 2016 report. Strasbourg, France: EDQM Publishers; 2021, EMCDDA. The elimination barometer for viral hepatitis among PWID in Europe, 2021. Available at: https://www.emcdda.europa.eu/publications/html/viral-hepatitis-elimination-barometer_en
Hepatitis B vaccination
WHO Target for 2020
95%
coverage with three doses of HBV vaccine
in countries that implement universal childhood vaccination
The hepatitis B vaccine has been instrumental in reducing the global incidence of hepatitis B among children under the age of five years [2]. In the EU/EEA, 27 countries recommend universal childhood vaccination against hepatitis B. Three countries do not have a national policy for universal vaccination (Denmark, Finland, and Iceland). Hungary has a nationwide school-based vaccination programme for hepatitis B that targets adolescents with two doses.
Data on vaccine coverage in 2020 were available from 23 countries. Of these, 11 countries (50%) have met the 2020 target of 95% coverage (Figure 4).
Some countries reported that coverage of the three doses of HBV vaccine has declined since 2019 which may be due to the COVID-19 pandemic and resultant public health measures [11, 12]. The declines reported ranged from 1-3%, with Romania (-3%), Bulgaria (-2%) and Croatia (-2%) reporting the largest declines [13]. While some countries were able to adapt services to continue vaccination coverage during the COVID-19 pandemic, other countries experienced interruptions to their vaccinations programmes and increased parental hesitancy towards vaccinations, negatively impacting vaccination rates [11, 12].
Coverage (%) of three doses of HBV vaccine (HB3) in EU/EEA countries that implement universal HBV vaccination in 2020
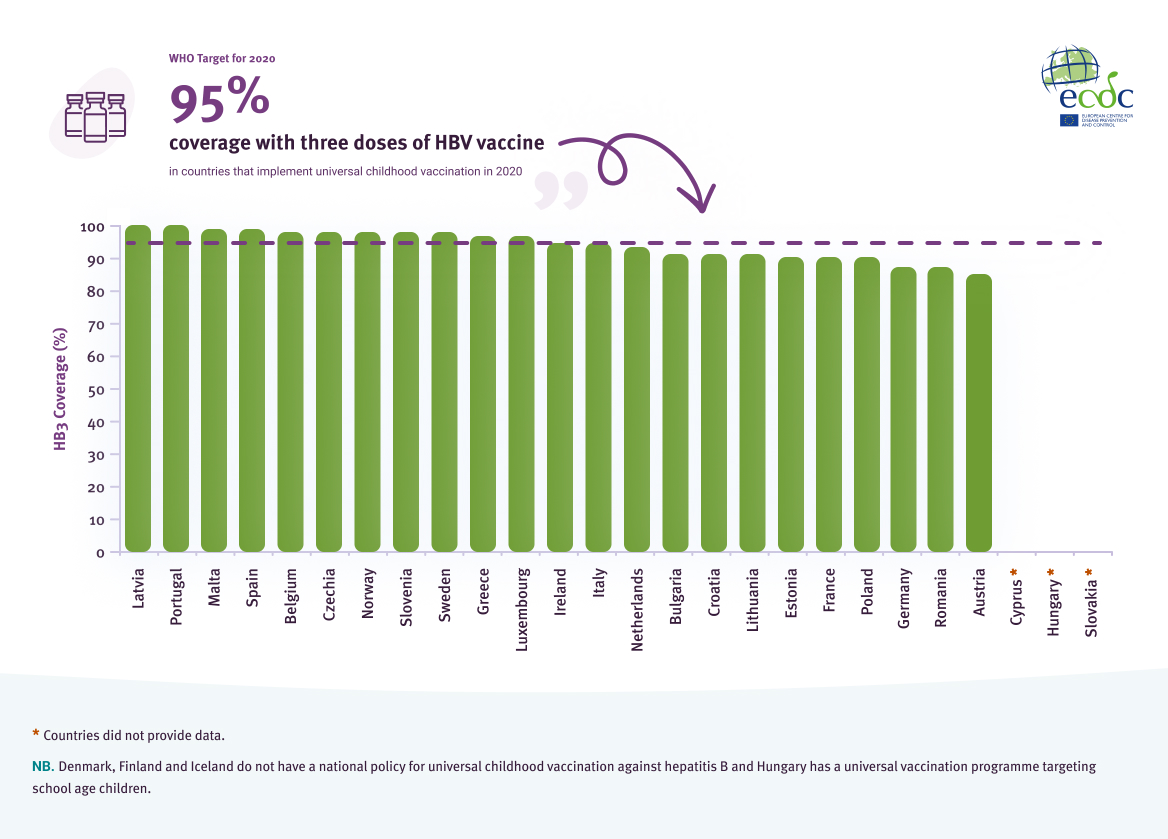
Source: WHO/UNICEF coverage estimates
Another important element to a hepatitis prevention strategy is enabling adults in key populations, such as PWID and healthcare workers, to access the HBV vaccine. Sixteen countries reported that they had an HBV vaccination policy or programme aimed at vaccinating PWID against HBV. However, there are limited data on vaccination coverage in this population, with only five countries reporting coverage rates ranging from 26% to 84%. The data provided mostly came from self-reported surveys, with only one country reporting that the data were from surveillance. Eighteen countries reported HBV vaccine programmes aimed at PWID in prisons [14].
Twenty-four countries reported that they have national HBV vaccination policies or programmes aimed towards healthcare workers. Seven of the 24 countries reported that the HBV vaccine was mandatory for all healthcare workers, while an additional two reported that it is was mandatory for healthcare workers ‘at risk’ of contracting HBV[2]. Seventeen countries reported that the HBV vaccine was offered to all or some healthcare workers. Eight countries reported estimates of HBV vaccination coverage among eligible healthcare workers, which ranged from 15.1% to 100% (Figure 5). The data came from a range of sources, including surveillance, surveys, and occupational health registries.
Hepatitis B vaccination coverage among people who inject drugs and healthcare workers, 2021
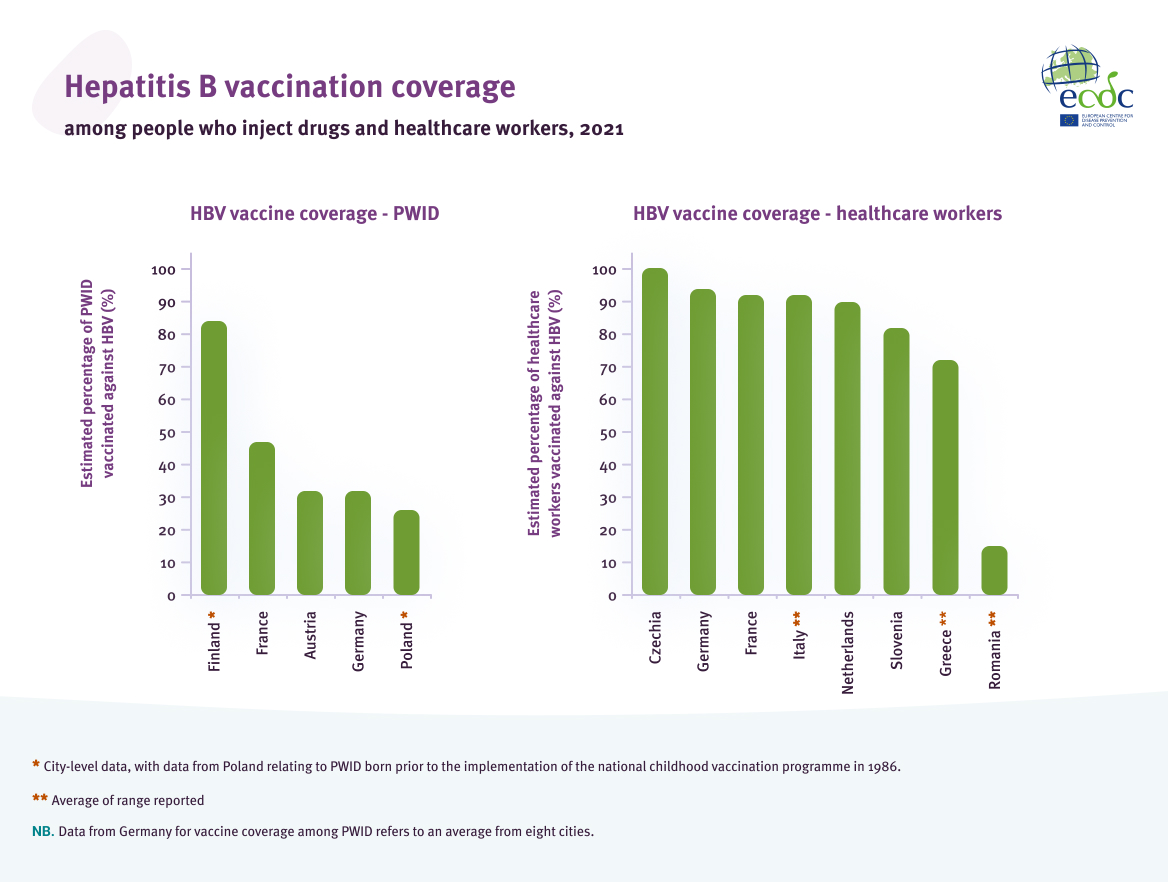
Source: Data reported from EU/EEA countries through ECDC hepatitis monitoring survey, 2021
Prevention of vertical transmission
WHO Target for 2020
90%
coverage with timely HBV birth dose vaccine
or, 90% coverage with screening in pregnant women and 95% coverage with post-exposure prophylaxis in infants born to infected mothers
In the EU/EEA, rates of vertical transmission of HBV are low, with national vertical transmission rates ranging from 0 – 0.5%. However, data availability on the rates of vertical transmission are limited, with only four countries able to provide national-level data (Table 1).
National-level rates of vertical transmission of hepatitis B virus in four countries in the EU/EEA
| Country | Rate of Vertical HBV Transmission (%) | Year(s) of Data Collection |
|---|---|---|
| Denmark | 0.1 | 2012 – 2021 |
| Greece | 0 | 2020 |
| The Netherlands | 0.5 | 2003 – 2011 |
| Slovenia | 0 | 1997 – 2020 |
Source: Data reported from EU/EEA countries through ECDC hepatitis monitoring survey, 2021
There are different strategies which countries can implement to reduce the risk of vertical transmission, including antenatal screening combined with post-exposure prophylaxis and universal newborn vaccination.
Data on coverage of antenatal screening programmes for pregnant women were available from 13 of the 25 countries reporting universal screening programmes. Of the 13, 10 countries (77%) met the 2020 target of 90% coverage of antenatal screening (Figure 6).
Coverage of antenatal screening in EU/EEA countries that implement universal antenatal testing for HBV in 2020[3]
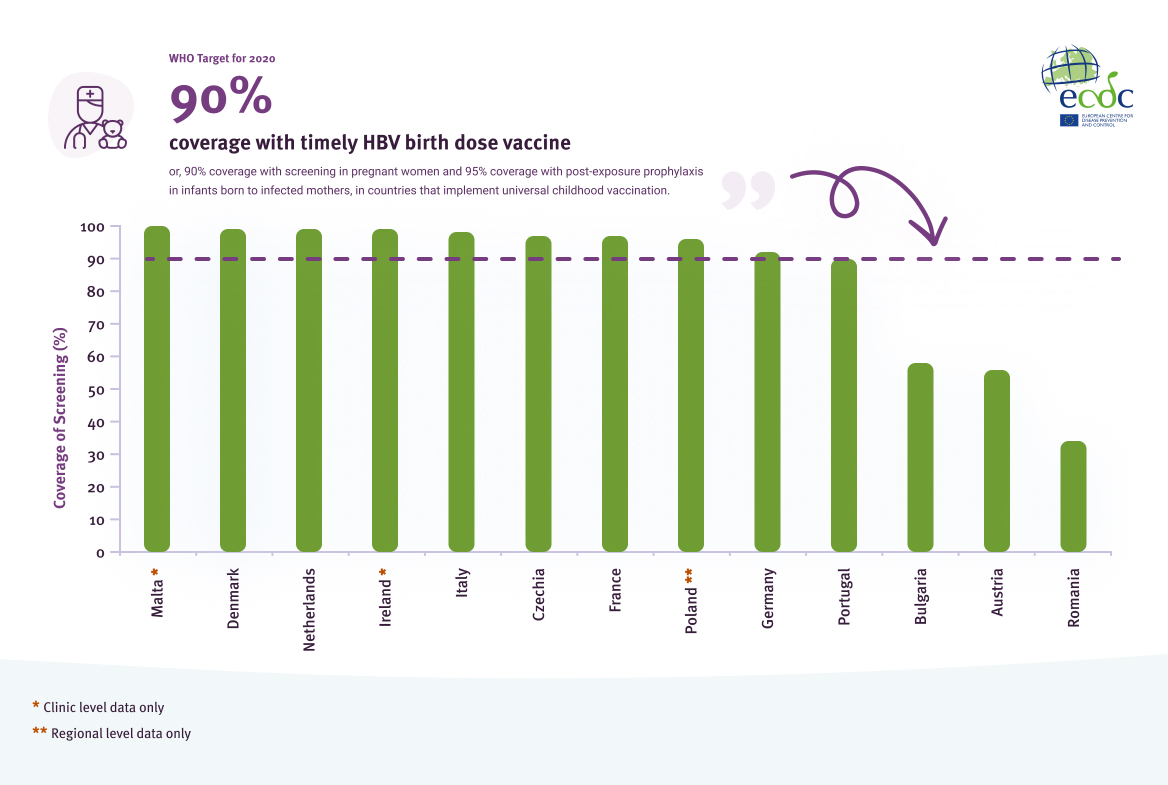
Source: Data reported from EU/EEA countries through ECDC hepatitis monitoring survey, 2021
Of the 25 countries with universal antenatal screening, 24 (96%) reported that there was a policy on postexposure prophylaxis for children born to mothers who have HBV (Figure 7)[4]. A total of 21 of the 25 countries (84%) reported a policy for hepatitis B immunoglobulin (HBIg), 17 (68%) reported antiviral treatment for mothers identified with HBV infection and one country (4%) reported antiviral treatment for the infant.
Measures included in national policies on post-exposure prophylaxis for children born to mothers who have HBV in countries with universal antenatal screening in the EU/EEA in 2020
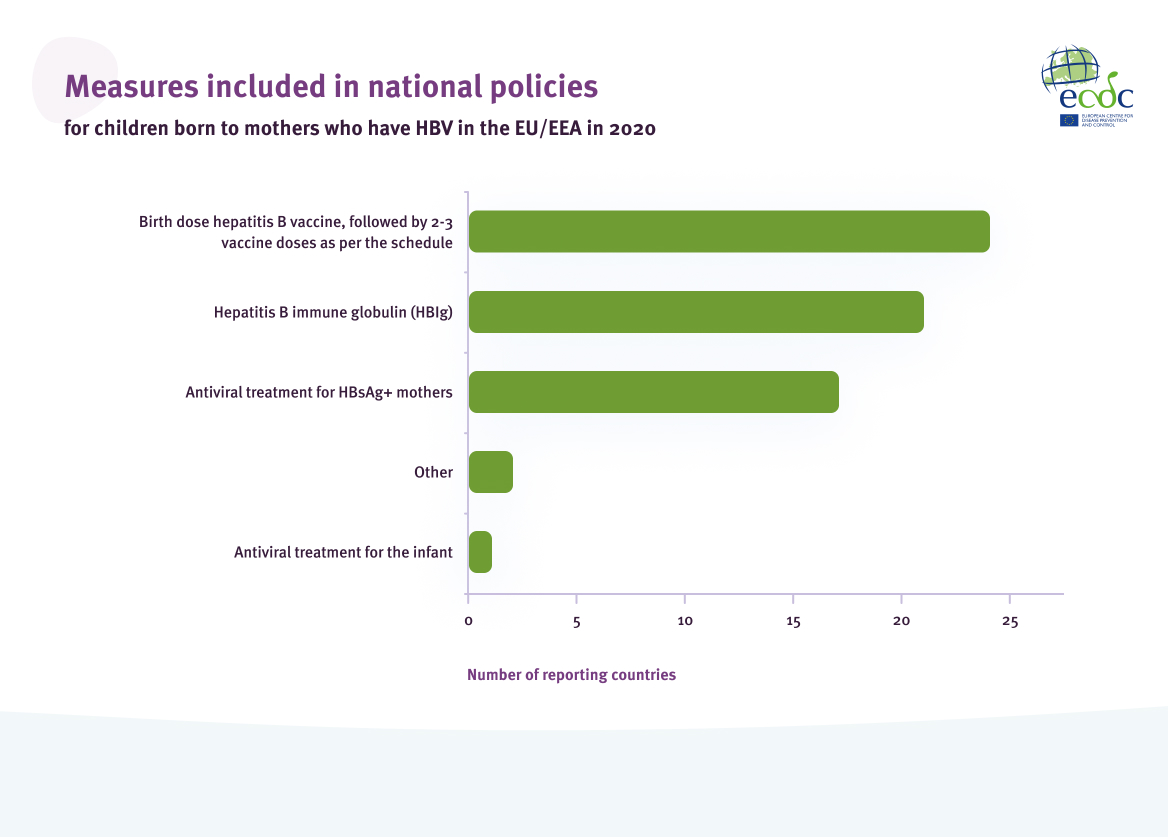
Source: Data reported from EU/EEA countries through ECDC hepatitis monitoring survey, 2021
Of the 24 countries with policies on post-exposure prophylaxis for infants born to mothers who have HBV, all (100%) reported that their policies included a birth dose of the hepatitis B vaccine, followed by two to three vaccine doses as per the childhood vaccination schedule. Only six countries were able to provide coverage of the birth dose vaccine for infants born to mothers who have HBV, with coverage ranging from 82% to 100% (Figure 8).
Coverage (%) of birth dose vaccine for infants born to mothers who have HBV in the EU/EEA
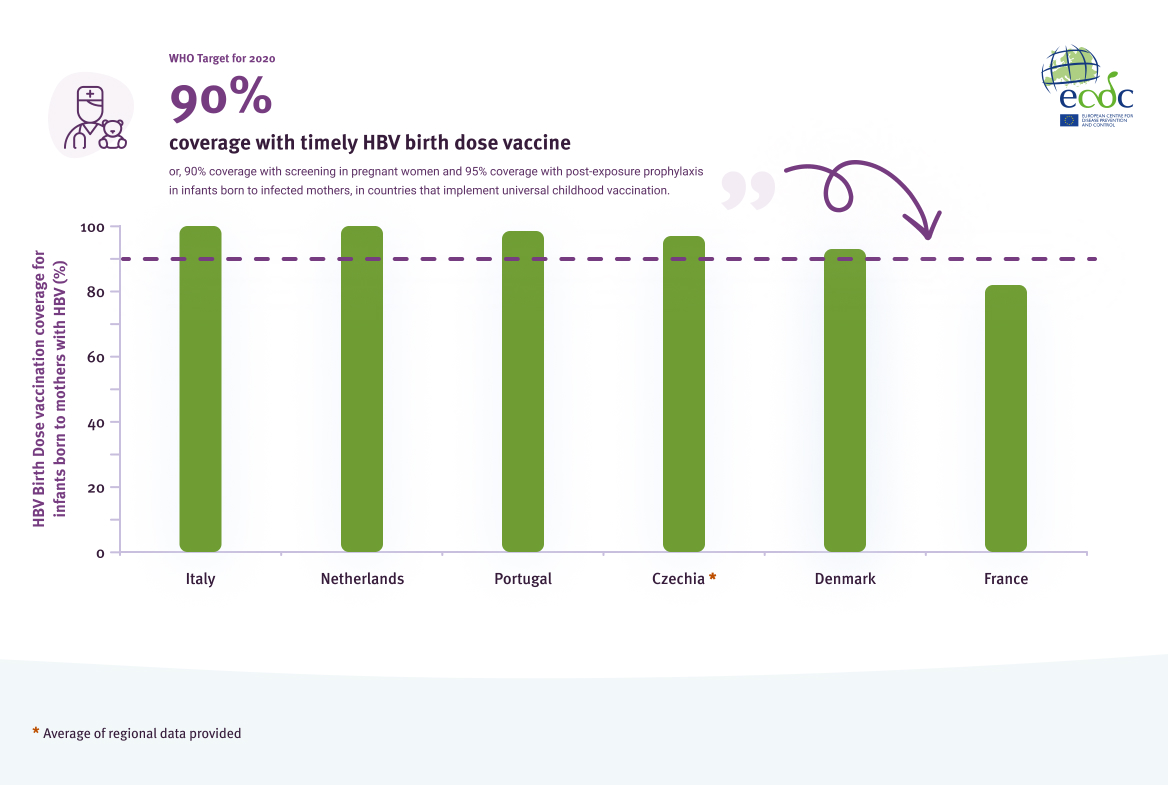
Source: Data reported from EU/EEA countries through ECDC hepatitis monitoring survey, 2021
While 24 countries provide a birth dose vaccine to infants born of HBV positive mothers, only five countries in the EU/EEA provide a universal birth dose of the HBV vaccine. Four (80%) of the five countries reached the 2020 target of 90% coverage (Figure 9).
Coverage (%) of birth dose vaccine in EU/EEA countries that implement universal newborn vaccination in 2020
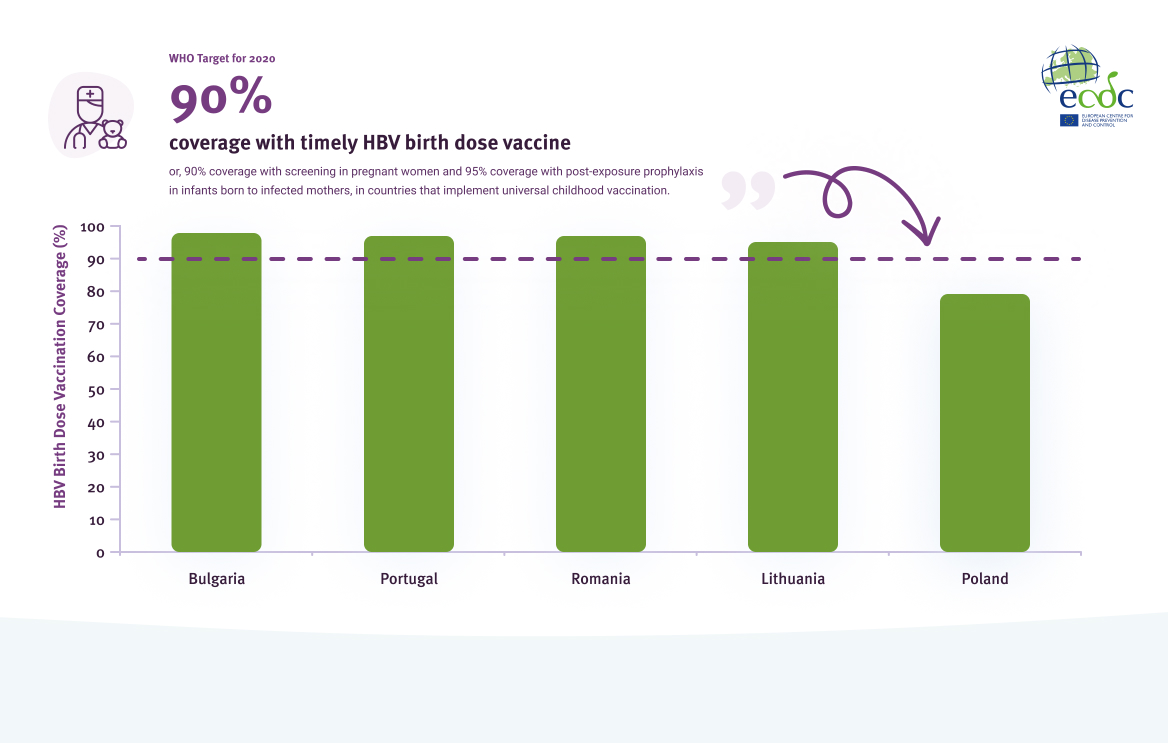
Source: World Health Organization. WHO/UNICEF estimates of national immunization coverage. Geneva, Switzerland: WHO; 2021. Available here.
Blood safety and haemovigilance
WHO Target for 2020
100%
of blood donations screened using quality-assured methods
All donated blood tested with nucleic acid test (NAT) screening methods for hepatitis B and C virus; all donated blood donated from non-remunerated donors
Haemovigilance refers to the surveillance of the blood transfusion chain, including efforts to monitor and evaluate adverse events associated with the blood supply and transfusion service. All EU/EEA countries screen blood donations using quality assured methods according to EU standards and have haemovigilance systems in place with donations tested at least with serological methods for HBV and HCV infections [15]. However, only 13 of 22 (59%) responding countries reported screening all blood donations for HBV and HCV using nucleic acid amplification testing (NAT). Additionally, 21 of 22 countries reported that all donated blood is from voluntary, non-remunerated donors (Figure 10).
The prevalence of HBV and HCV infections among first time blood donors in the EU/EEA is low, with a median HBV prevalence of 82 per 100 000 first-time donors (range: 0 – 3 840 per 100 000) and a median HCV prevalence of 50 per 100 000 first-time donors (range: 4 – 4 527 per 100 000) [14]. Additionally, the number of transfusions associated HBV and HCV infections reported by EU/EEA countries is very low. No countries reported transfusion associated transmissions of HCV and only two countries reported transfusion associated HBV transmissions. Ireland reported one likely, probable, or confirmed transmission per 298 108 transfusions and Slovenia reported two likely, probable, or confirmed transmissions per 120 986 transfusions [15].
Proportion of whole blood donations from voluntary, non-remunerated sources (%) in EU/EEA countries, 2016

Source: EDQM (Council of Europe). The collection, testing and use of blood and blood components in Europe: 2016 report. Strasbourg, France: EDQM Publishers; 2021
Prevention among PWID
WHO Target for 2020
200 +
sterile injection equipment kits distributed per person per year
A comprehensive package of harm reduction services to all persons who inject drugs, including: at least 200 syringes distributed per PWID per year; at least 40% of opioid dependent PWID receive opioid substitution therapy (OST); and HBV and hepatitis A virus (HAV) vaccination
PWID are disproportionally affected by HBV and HCV infections due to shared injection equipment and drug paraphernalia. In Europe, there is a high prevalence of infection and ongoing transmission in this population, especially for HCV. High levels of needle exchange coverage and opioid substitution therapy are effective at reducing the risk of viral hepatitis transmission among PWID.
Fourteen countries provided data on the coverage of hepatitis prevention programmes aimed towards PWID. Of the 14, only two (14%) met both the syringe distribution target and the OST coverage target (Figure 11). Three countries (21%) met or exceeded the 2020 target of 200 syringes distributed per PWID per year. Additionally, three countries were within 15% of the 2020 syringe distribution target. Nine countries (64%) reported 40% coverage of more of OST.
Number of sterile syringes distributed per person who injects drugs and proportion of high-risk opioid users in opioid substitution treatment (OST), by country, 2019 or latest data
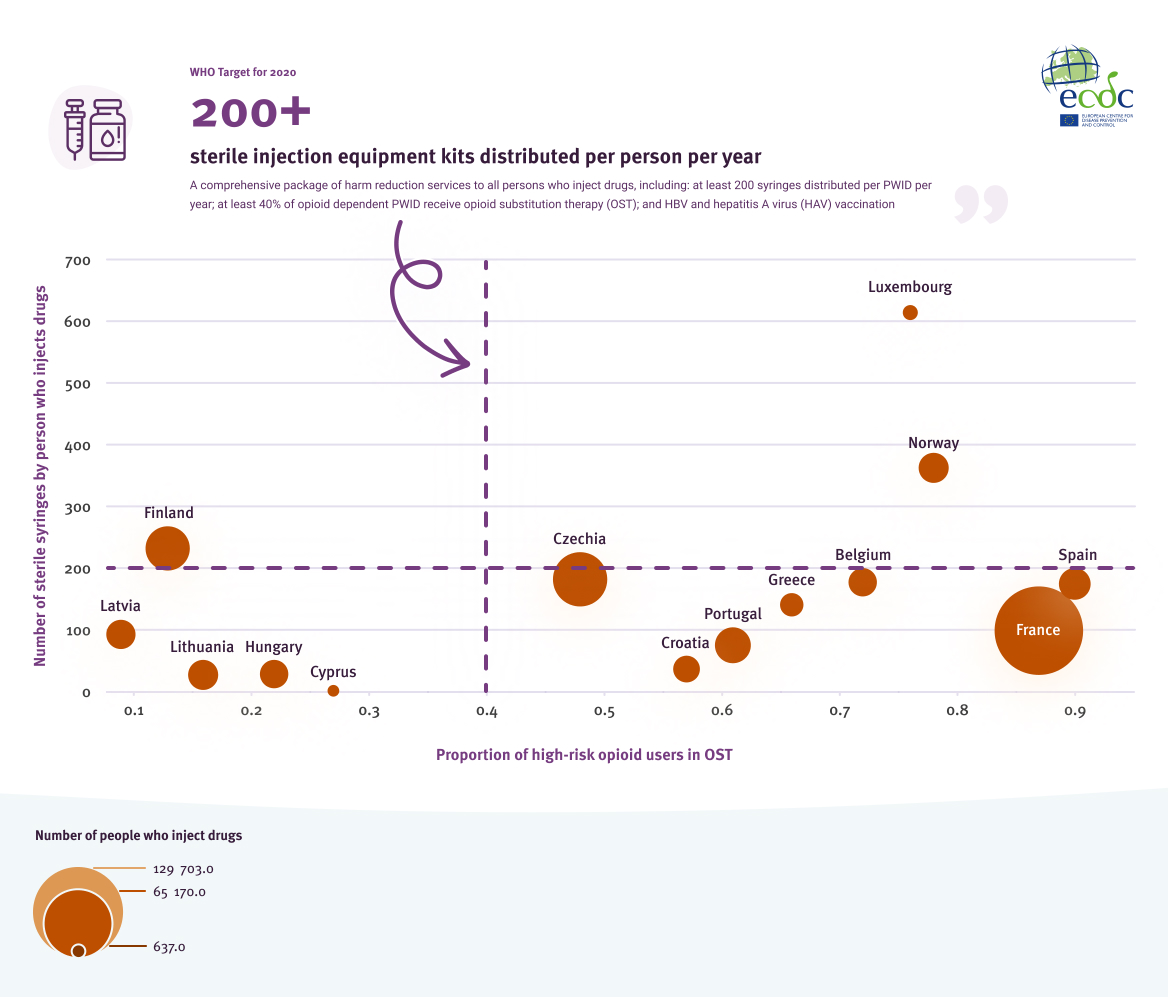
Source: EMCDDA. The elimination barometer for viral hepatitis among PWID in Europe, 2021
Treatment as prevention
With the discovery of effective direct-acting antivirals (DAAs) and the increased availability of hepatitis C treatment, treatment as prevention is an emerging tool to help prevent HCV transmissions [16]. Several studies have concluded that DAAs are effective tools to help progress towards HCV micro-elimination in key populations, including MSM, PWID and people in prisons [16, 17,18, 19]. In 2021, it was reported that DAAs are available in all countries in the EU/EEA [20]. However, civil society organisations in 37% of the countries reported that restrictions existed when accessing DAAs, including restricted access for certain populations, restricted access to those with insurance and restricted access based on genotype of infection, which may limit the potential to use DAAs as a prevention tool [20]. Moreover, data on numbers diagnosed and treated collected by ECDC from across EU/EEA countries indicate that a significant number of individuals with chronic hepatitis C remain undiagnosed and, while the data are incomplete, there is evidence that in some countries a large proportion of those diagnosed have not yet been treated [21].
Prevention of sexual and nosocomial transmission
WHO Target for 2020
50%
of injections administered with safety-engineered injection devices
integrated into broader infection prevention and control
Although nosocomial infections account for 12% of acute HBV transmissions and 5.3% of acute HCV transmissions[5], there are no readily available data on efforts to prevent infections in hospitals. Twenty-four countries reported that their HBV vaccination policies include healthcare workers, however only eight were able to provide the rate of vaccination among healthcare workers and these showed great variation in coverage. In order to have a comprehensive understanding of nosocomial HBV/HCV infections, further work is needed to determine how best to monitor nosocomial infections in hospitals and the coverage of prevention programmes and protocols, such as the usage of safety-engineered injection devices.
There are also limited data available on the coverage of preventative measures for the sexual transmission of HBV and HCV. Available data from the 2017 European Men-Who-Have-Sex-With-Men Internet Survey (EMIS- 2017) focus exclusively on MSM [22]. Public health authorities recommend that specific MSM are vaccinated against hepatitis B, such as MSM who are infected with HIV or HCV or MSM who have sex with multiple partners [22]. The results from EMIS-17 show that just under half (49%) reported they had been vaccinated against hepatitis B with a full course of vaccination and 26% did not know where to get hepatitis B vaccine (and were not vaccinated) [22]. Moreover, 17% reported that they did not know that there are vaccines available for hepatitis B (and/or A) [21]. This suggests a needs to increase awareness around hepatitis B and promotion of HBV vaccination within this population.
Additionally, harm reduction measures can also be used to reduce the risk of the sexual transmission of viral hepatitis. However, 22% of MSM reported that the sex they have is not as safe as they would like, which may be in part related to a lack of access to harm reduction measures, including measures targeting chemsex [22].
Data provided by EMIS-2017 suggest a lack of awareness of hepatitis vaccination and an increased need for harm reduction services in order to reduce transmission of viral hepatitis among MSM, for whom the mode of transmission (sexual or through injecting drug use) may be unclear [22]. However, in relation to the prevention of heterosexual transmission of viral hepatitis, which is the leading reported cause of transmission for hepatitis B, further work is needed across the EU/EEA to obtain a clearer overview of efforts in this area.
Conclusions
Action is required to improve efforts to prevent new hepatitis B and C infections and get the region on track to reach SDG 3.3, combatting viral hepatitis.
The epidemics of HBV and HCV in the EU/EEA are complex and dynamic, with evidence of ongoing transmission of infections and extremely high prevalence in some population groups. There remain significant data gaps on HBV and HCV epidemiology and available prevention programmes and these gaps present major challenges to monitoring progress towards elimination targets. ECDC is ready to support countries in their efforts to improve the availability and quality of their data.
While data are lacking from many countries, according to available data, many countries in the EU/EEA have not met all of the 2020 European Action Plan targets for viral hepatitis prevention.
Currently, there is suboptimal HBV vaccine coverage across EU/EEA countries for programmes targeting children as part of the primary schedule as well as among key adult populations at risk of infection. Programmes for the prevention of vertical transmission for HBV infection are not well monitored and data from these programmes should be collected routinely to assess their delivery and identify any gaps in services that need addressing. Given the importance of harm reduction services for the prevention of transmission of blood-borne infections among PWID and the high burden of infections among this group, there is an urgent need to strengthen and expand prevention and testing services aimed at this population, as there is evidence of suboptimal implementation in many countries across the EU/EEA. Vaccination efforts targeting MSM should also be reviewed and strengthened where necessary.
Priority areas for action
Priority 1
Countries should consider strengthening their viral hepatitis prevention strategies to get on track towards eliminating viral hepatitis as a public health threat in Europe.
Priority 2
A lack of robust, reliable data on hepatitis B and C prevention is a significant barrier to monitoring progress towards the WHO European Action Plan targets. There is an urgent need for better systems to monitor progress at the national level.
Improved data collection for monitoring is a top priority, especially in the following areas:
- HBV and HCV prevalence in general populations and among key populations, such as PWIDs and people in prisons, along with sizes of key populations to better understand the numbers infected.
- Vaccination policies and coverage in key adult populations, including PWID, people in prisons, people living with HIV, MSM, and healthcare workers.
- Data on prevention of hepatitis via sexual and nosocomial transmission routes, with the development of key indicators for which data can be easily collected.
- Coverage of programmes to prevent mother to child transmission.
Priority 3
Countries should strengthen the implementation of harm reduction programmes aimed at PWID and sexual health programmes aimed at MSM, along with improved hepatitis prevention (e.g. HBV vaccination), testing, and treatment in community settings.
Priority 4
Efforts to maximise coverage of HBV vaccination for children should be prioritised, especially given the concerning declines in vaccine coverage observed in some countries in recent years.
Priority 5
The gaps in data and in the programmes for the prevention of vertical transmission, including antenatal screening and birth dose vaccination for babies born to mothers with HBV infection should be addressed to minimise the likelihood of any transmission occurring through this route.
[2] One country defined ‘at risk’ as employees inside and outside the healthcare system who have a significant risk of infection transmission and stab wounds; employees in housing units for the mentally handicapped where one or more residents has hepatitis B; and employees in day-care institutions with known hepatitis B. The other country did not provide a definition of at risk.
[3] Year of country data collection: Malta (2020), Denmark (2019), the Netherlands (2017), Ireland (2020), Italy (2008-2009), Czechia (2020), France (2016), Poland (2016), Germany (2011-2015), Portugal (2020), Bulgaria (2020), Austria (2019), Romania (2020).
[4] Two countries reported ‘other’ policies. These policies were: combination therapy of the birth dose vaccine and provision of HBIg (active and passive vaccination) and HBV testing on the infants first birthday.
[5] Where the transmission route is known and reported.