Factsheet about Marburg virus disease
Disclaimer: The information contained in this factsheet is intended for the purpose of general information and should not substitute individual expert advice or the judgement of healthcare professionals.
Marburg virus disease (MVD), formerly known as Marburg haemorrhagic fever, is a severe disease in humans caused by Marburg marburgvirus (MARV). Although MVD is uncommon, MARV has the potential to cause epidemics with significant case fatality rates. All recorded MVD outbreaks have originated in Africa. MVD is not an airborne disease and is considered not to be contagious before symptoms appear. Direct contact with the blood and other body fluids of infected people and animals or indirect contact with contaminated surfaces and materials like clothing, bedding and medical equipment is required for MARV transmission [1]. As a result, if proper infection prevention and control precautions are strictly followed, the risk of infection is regarded as minimal. There is no approved vaccine for MVD; however, several candidate MVD vaccines are in clinical trials.
Case definition
MVD in humans is a notifiable disease at the EU/EEA level [2]. The case definition for viral haemorrhagic fevers is defined according to the Commission Implementing Decision (EU) 2018/945 of 22 June 2018 [3].
The pathogen
Marburg viruses are filamentous, enveloped, single-stranded, negative-sense RNA viruses that belong to the family Filoviridae, genus Marburgvirus. There is a single species Marburg marburgvirus, that includes two viruses: Marburg and Ravn virus with approximately 20% genetic divergence [4]. MARV Musoke, Angola, Ci67, Ozolin, Popp, Ratayczak and Voege are MARV variants with fewer genomic differences [5]. Both Marburg viruses cause clinically indistinguishable disease (MVD) and are highly lethal human pathogens that have been linked to several epidemics of haemorrhagic fever in Africa.
The MARV genome encodes seven structural proteins with a different role in the MVD pathogenesis. Viral RNA is associated with the nucleoprotein (NP), viral protein 30 (VP30), VP35 and the L-polymerase (L) which form the nucleocapsid. A matrix (composed of VP40), VP24 and a lipid envelope with surface glycoprotein (GP) spikes surrounds the ribonucleoprotein [4,6]. Cell and tissue tropism and virus‑cell membrane fusion are determined by MARV GP. In addition, GP may play a role in immune evasion by counteracting the antiviral effects of tetherin, an antiviral interferon (IFN)-stimulated protein that inhibits viral spread [6,7]. VP40 is a virulence factor that counteracts the innate immune response in addition to being a major matrix protein [8]. The main function of VP40 in MARV immunopathology is to supress host cell responses to IFN signalling [9]. The VP35 is a multifunctional virulence factor that facilitates immune evasion by impairing the IFN response and is important for viral RNA synthesis [7]. The minor matrix protein VP24 blocks cellular response to IFN [7]. The L protein mediates genome replication and transcription [4].
MARV is classified as a risk group 4 (RG-4) pathogen and requires stringent containment and barrier protection measures for laboratory personnel, as well as for anyone caring for potentially infected patients or deceased bodies [10].
Clinical features and sequelae
The incubation period lasts from five to 10 days (range 3–21 days), and is most likely related to the infectious dose and the route of infection [11]. Transmission does not occur during the incubation period.
The clinical course can be divided into three phases: the first generalised phase (days 1–4), early organ phase (days 5–13), followed by either a late organ or a convalescence phase (days 13+). In this stage, supportive care can maintain the patient until the virus is eradicated spontaneously [12]. Survivors rarely show the most severe symptoms of the disease and may never reach the late organ phase [13]. The onset of MVD is abrupt, with non-specific, flu-like symptoms such as a high fever (usually 39–40°C), severe headache, chills, myalgia, prostration, and malaise. In 50–75% of patients, rapid debilitation, marked by gastrointestinal symptoms such as anorexia, abdominal discomfort, severe nausea, vomiting, and diarrhoea, occurs within 2–5 days. The intensity of the disease increases on days 5–7, with a maculopapular rash and symptoms of haemorrhagic fever, such as petechiae, mucosal and gastrointestinal bleeding, and bleeding from venipuncture sites. Neurological symptoms (disorientation, agitation, seizures, and coma) can occur in later stages of the disease [14]. Joint pain, uveitis, orchitis, recurrent hepatitis, pericarditis and mental dysfunction have been documented as complications during convalescence, which can be slow [13].
Disseminated intravascular coagulation, lymphopenia and thrombocytopenia typically appear within a week after the disease onset. Patients either recover with supportive therapy or die from dehydration, internal bleeding and multiorgan failure 8–16 days after symptom onset. Early supportive care improves survival. MVD survivors have experienced various sequelae, including exhaustion, myalgia, hyperhidrosis, skin desquamation, and hair loss [11].
Because of the lower case fatality rate linked with the initial outbreak in Europe (24%), MARV was initially thought to be less virulent than Ebola virus (EBOV) [11,15]. However, subsequent larger MVD outbreaks have shown extremely high case fatality rates: 83% in the Democratic Republic of the Congo [16] and 88% in Angola [17]. Although factors associated with these increased fatality rates compared to the European cases remain unexplained, the epidemiological data from the Uige outbreak have indicated that the Angola strain may be more virulent than other MARV strains [13].
Epidemiology
In 1967, two outbreaks of VHF occurred simultaneously in Marburg, Germany, and in Belgrade, Serbia (historically Yugoslavia) among laboratory workers in Europe working with tissues of African green monkeys imported from Uganda, as well as among medical personnel who cared for the laboratory workers. Nine people of the 37 cases died, with some cases spreading through household or nosocomial contact. MARV was named after the German city where it was first characterised [11,15].
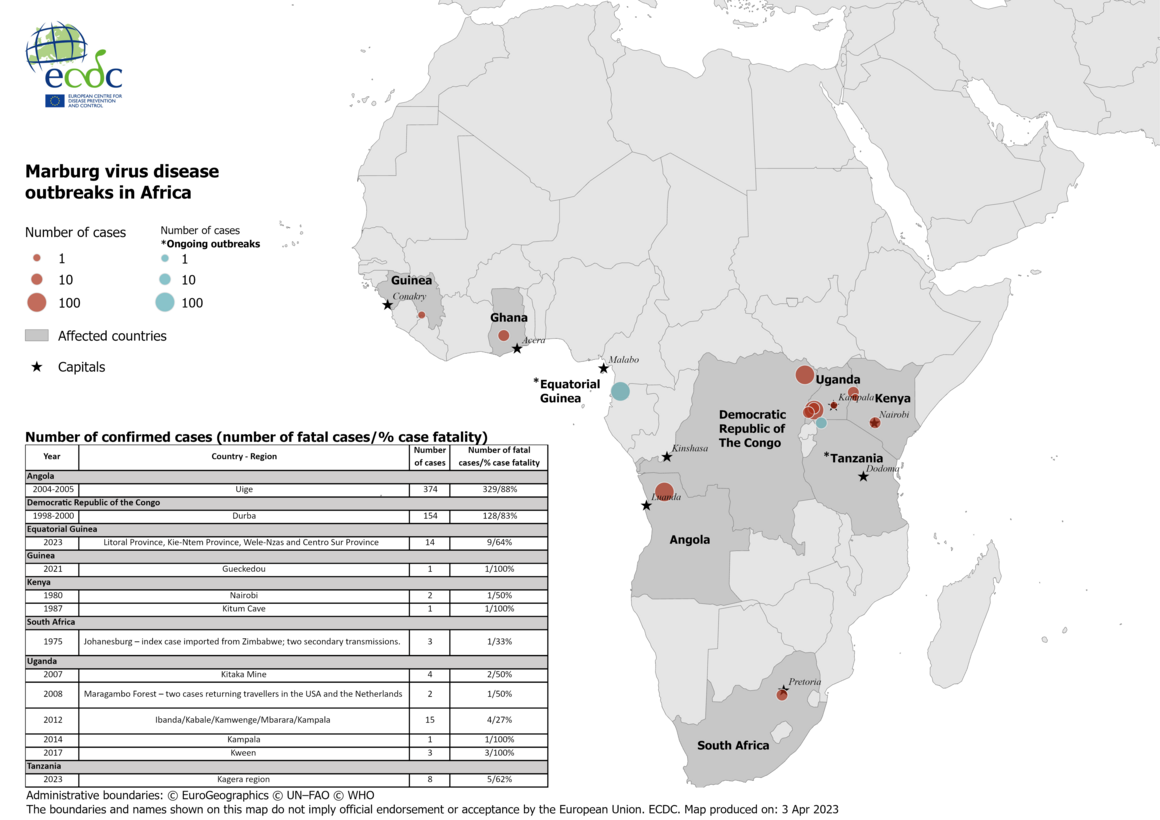
Nearly 600 MVD cases have been reported since then in outbreaks in Uganda, the Democratic Republic of the Congo (DRC) and Angola [16-18] (Figure 1). The first large outbreak, in Durba, DRC (1998–2000) occurred among gold miners and involved 154 cases and 128 deaths (case fatality rate 83%) [16]. The largest outbreak to date, in Uige Province, Angola (2004–2005) resulted in 374 cases and 329 deaths (case fatality rate 88%) [17]. In a 2012 MVD outbreak in Uganda, a total of 26 confirmed and probable cases were notified, 15 (58%) of which were fatal. The outbreak started in Ibanda District and expanded to at least four other districts including Mbarara, Kabale, Kamwenge and Kampala [18]. In 2017, a family cluster involving four cases (3 confirmed and one probable) occurred in Uganda (Kween District) [19]. Sporadic cases of MVD were also detected in South Africa (Johannesburg, 1975), Kenya (Western Province, 1980 and 1987), Uganda (Kamwenge Districts in 2007, Kampala in 2014) and Guinea (Gueckedou, 2021) [20-24] (Figure 1). The 1975 cases in Johannesburg were one person who had recently travelled to Zimbabwe and two secondary cases – a traveling companion and a nurse [20]. The 2021 case in Gueckedou, Guinea, was the first known MVD case in West Africa [23]. In 2022, three cases were reported in the Ashanti region of Ghana [25]. In February 2023, Equatorial Guinea confirmed the first MVD outbreak in the country. In this ongoing outbreak, communities in four provinces (Litoral Province, Kie-Ntem Province, Centro Sur Province, and Wele-Nzas province) have been affected so far. Until 30 March 2023, 14 confirmed cases including nine deaths were reported. In March 2023, the first MVD outbreak in Tanzania was reported in the Kagera region. Until 30 March 2023, eight cases including five deaths were reported in this ongoing outbreak.
In 1990, an incident of MARV infection occurred in the former Union of Soviet Socialist Republics (USSR), when a researcher became infected after contact with an archived serum sample from a MARV-infected animal [25]. (Figure 1).
In Europe, an imported fatal case of MVD was detected in July 2008 in a Dutch woman who developed symptoms less than two weeks after she returned from vacation in Uganda. Exposure most likely occurred in the Python Cave, Maragambo Forest, which is known for its colony of Egyptian fruit-eating bats (Rousettus aegyptiacus) that have been found positive for filoviruses, including MARV in other sub-Saharan African areas [27]. No MVD cases have been reported in the EU/EEA thereafter. In addition, in 2008, MVD was recorded in a US tourist returning from a 2-week safari in Uganda. Ten days before to the onset of symptoms, the patient from Colorado had visited the same cave as the Dutch tourist mentioned above [28].
The geographical range where MARV has been detected in bats (arid woodlands of Equatorial Africa) is more widespread than recorded human outbreaks and they coincide with the presence of the Rousettus aegyptiacus bats [29].
Figure 1. MVD outbreaks in Africa: number of confirmed cases (number of fatal cases/ % case fatality)
Transmission
The majority of natural MVD outbreaks have been connected to human entry into bat-infested mines and caves, suggesting that bats play a key role in MARV transmission. In 2007, MARV was isolated from R. aegyptiacus bats giving strong evidence that this species represents a major natural MARV reservoir (26). However, the virus maintenance and transmission within bat populations remains unknown. In addition, it is not clear how MARV transmission from bat reservoir to humans occurs.
Once an individual is infected, interhuman transmission occurs via direct contact (broken skin or mucous membranes) with the blood and other body fluids (urine, saliva, faeces, vomit, breast milk, amniotic fluid, and semen) of infected people, or indirect contact with contaminated surfaces and materials such as clothing, bedding, and medical equipment. In addition, infection may occur in relation to the burial of infected individuals [29]. Contact with dead or living infected animals, including bushmeat (e.g. monkeys, chimpanzees, forest antelopes, and bats) can also be a source of infection [30]. The consumption of bushmeat has been associated with EBOV epidemics. Some bushmeat species, such as chimpanzees and forest antelopes, are susceptible to virus replication and die from infection; they are therefore considered to be intermediate hosts, rather than the reservoir species [31]. Filovirus testicular persistence as a putative transmission mechanism is causing increasing concern. Experimental studies showed that male monkeys developed sustained MARV infection of the seminiferous tubules, an immune-privileged site [32]. The first reported case of potential sexual transmission occurred during the MARV outbreak in 1967. The wife of a male patient become ill two months after her husband recovered from the disease, which was substantiated by detection of MARV antigen in the husband’s semen [33]. At the same time, the seminal fluids of nine other convalescents were analysed for the presence of MARV, but no virus or viral antigen was detected [34]. Although the virus was detected in the semen of some patients who recovered from MVD up to 203 days after the onset of disease, there is no strong evidence for sexual transmission of MARV [35].
To date, only a few cases of MVD in pregnancy have been reported. Filovirus infection is generally more severe in pregnant than in non-pregnant women, possibly because of altered immune function or involvement of the placenta [36]. High viral titres have been found in placental tissue, indicating that haematogenous propagation of the virus through the placenta is the most prevalent route of foetal infection, according to case reports [37]. Despite high fatality rates in pregnancy, there is no evidence that pregnant women are more susceptible to filovirus infection than others [38]. Pregnant women with MVD are more likely to have a spontaneous abortion or a stillbirth [29]. There is very little information on the neonatal outcomes following MVD infection. Several clusters of MVD have been reported in infants, and some of these infants presented surprisingly mild symptoms [39].
The presence of MARV in blood and consequently organs and tissues of infected or recovered individuals indicates that transmission via transfusion and transplantation is possible. However, transmission of MARV through substances of human origin has not been reported.
Filoviruses can survive in liquid or dried material for many days [40]. They are inactivated by gamma irradiation, heating for 60-75 minutes at 60°C or boiling for five minutes, and are sensitive to lipid solvents, sodium hypochlorite, and other disinfectants [41,42].
Diagnostics
Clinical diagnosis of MVD can be difficult, since many of the signs and symptoms are comparable to those of other infectious diseases such as malaria, typhoid fever and dengue, as well as other viral haemorrhagic fevers that may be endemic in the area, such as Lassa fever or Ebola virus disease [43].
Depending on the time course of the infection, diagnostic procedures for MVD include virus isolation, reverse transcription-polymerase chain reaction (RT-PCR), antigen detection, serology, and immunohistochemistry [44,45].
Virus isolation is a sensitive approach for diagnosis of MVD. MARV propagates well in different cell lines, but Vero cells and Vero E6 cells are most frequently used. Virus isolation should be performed in a BSL-4 laboratory, which often have limited availability; as a result, this is not used as a routine diagnostic method for MVD [46].
Molecular methods (RT-PCR, nested RT-PCR, real-time quantitative RT-PCR) targeting NP, L and GP genes have been shown to be sensitive, specific, and effective in MVD diagnosis [44,45]. As the GP is strain-specific, it may be used for differentiation between infecting species, whereas the VP40 and NP are more conserved. However, the sensitivity of the RT-PCR systems used in different laboratories varies. The main limitation of the majority of the available nucleic acid amplification tests is their limited strain coverage, which may potentially result in false negative results [47].
Since high virus titres are present in blood and tissues, antigen detection is suitable for diagnosis in early stages of MVD. The antigen-capture enzyme-linked immunosorbent assay (ELISA) targets the proteins NP, VP40, and GP [45,48].
Serological methods include ELISA and indirect immunofluorescence assay (IFA). The recombinant proteins rGP and rNP were confirmed to be useful as antigens for detection of MARV IgM and IgG antibodies. The IgM-capture ELISA has been shown to be a very useful diagnostic tool for MVD. Detection of MARV IgM antibodies indicates recent infection and can be detected as early as 2–4 days after symptom onset. MARV IgG antibodies can be detected 8–10 days after symptom onset and persist for up to two years after infection [47]. Although serology can be used for MVD confirmation, it should be kept in mind that negative serology is not exclusive, since filovirus-infected individuals frequently die without a development of humoral immune response [44,45]. As IgM response is being succeeded by IgG in filovirus infection survivors, negative MARV serology in patients who recover from a haemorrhagic fever could exclude MVD [48].
Histological techniques, including antigen detection by immunohistochemistry, are sensitive approaches, especially for post-mortem diagnosis [45].
There are only a few commercially available diagnostic tests for MARV. Samples from MVD patients should be handled in biosafety level 3 (e.g., RT-PCR and ELISA on non-inactivated samples) or level 4 laboratories (virus isolation) under strict biological containment circumstances. RT-PCR and ELISA testing of inactivated samples can be performed at BSL-2 laboratory facilities [10].
Case management and treatment
There is no specific antiviral treatment for MVD. Supportive therapy such as intravenous fluids, electrolyte replacement, supplemental oxygen, as well as blood and blood products replacement, may improve the clinical outcome significantly [50].
Immunotherapeutics, phosphorodiamidate morpholino oligomers, lipid-encapsulated short interfering RNAs, small molecule inhibitors, interferons, and antiviral nucleoside analogues are among the pharmaceuticals being developed to combat MARV. Favipiravir and remdesivir appear to be advantageous in non-human primates, but there is no evidence that they would be beneficial in humans. Galidesivir has been shown to be effective against MARV for up to 24 hours; phase 1 results are still awaiting [51]. Ribavirin, a broad-spectrum synthetic guanosine analogue, with virustatic activity against a variety of DNA and RNA viruses, and IFN have shown little benefit in countering MARV infection [13].
Public health control measures
The main goal of MVD outbreak control is to interrupt direct human-to-human transmission. Early detection and systematic rapid isolation of cases, timely contact tracing and close monitoring of those at risk, proper personal protection, safely conducted burials, and better community awareness about infection risk factors are all part of the outbreak control strategy. The isolation of infected patients combined with appropriate infection prevention and control measures has been shown to effectively stop the spread of EBOV and MARV in previous outbreaks [1,52].
All individuals working in the isolation area, laundering potentially infected linens, disinfecting items or houses, transporting patients, or providing safe burials must wear personal protective equipment [52]. Barrier nursing procedures, such as the use of gowns, gloves, masks, and face shields or goggles to avoid contact with blood or body fluids, are the most important infection prevention and control protocols for MARV [43,51].
Early and culturally appropriate community engagement and social mobilisation are critical for supporting outbreak response activities and increasing affected populations' knowledge of MARV risk factors as well as personal protective measures, particularly regarding practicing safe and dignified burials.
It is advisable to avoid habitats that may be populated by bats, such as caves or mines in areas/countries where MVD has been reported, as well as any form of close contact with wild animals, including monkeys, forest antelopes, rodents, and bats, both alive and dead, and manipulation or consumption of any type of bushmeat.
Infection control, personal protection, and prevention
Organised and collaborative interventions based on case identification and surveillance, case management and isolation, social/community mobilisation and education should decrease filovirus transmission. As soon as the diagnosis of MVD is suspected, patients should be separated in a single room with a separate bathroom according to VHF protocol, and appropriate measures should be performed.
Healthcare workers (HCWs) and caregivers who come into close contact with patients are at the greatest risk of contracting MVD because they are more likely to be exposed to infectious blood and body fluids. Laboratory workers are also at risk [30]. Barrier nursing strategies must be implemented to reduce the risk of infection among HCWs. Aerosol-generate procedures should be avoided. The use of needles and other sharp objects should be avoided as much as possible. Prior to disposal, any item in the isolation ward, including human excreta, must be disinfected with a bleach solution at a concentration of 1:10 [52].
As long as MARV persistence in semen has not been ruled out, recovered male patients are advised to practice safe sex, at least 12 months after clinical recovery according to WHO, unless their semen has tested negative on two different occasions [53]. No time interval between the two tests has been defined.
All contact with the patient’s body or fomites should be avoided during the burial process. Decontamination of the body using a 1:10 bleach solution and placement in a body bag are standard burial procedures. The body bag is then closed and the outside of the bag is similarly decontaminated [52].
There is no approved vaccine for MVD to date. Several candidate MARV vaccines are in Phase 1 clinical studies (cAd3, MVA-BN-Filo, and MARV DNA) [50,53,54]. For EBOV, several adenovirus-based vaccines have been tested, but there have been few studies for MARV. The most commonly used vector for GP vaccines is recombinant adenovirus serotype 5 (rAd5). Five filovirus antigens are used in the complex adenovirus (CAdVax) platform. These includes three GPs: EBOV, Sudan virus (SUDV) and MARV (Ravn, Musoke and Ci67 strains), and two NPs: EBOV and MARV-Musoke [56]. A Phase 1a clinical trial of a chimpanzee adenovirus serotype 3 vector vaccine (cAd3) MARV vaccine is currently ongoing. EBOV, SUDV and MARV GPs and Tai Forest NP are included in the modified vaccinia Ankara vector vaccine (MVA-BN-Filo), which is in Phase 2 clinical trial [54]. Sustained EBOV GP immunity was observed after either primary followed by alternate boost, but response to MARV antigens has not yet been evaluated [57]. Although DNA vaccines have the potential to produce humoral and cellular immunity in non-human primates, these have shown low immunogenicity in clinical trials. In cynomolgus macaques, MARV-Musoke GP and MARV-Angola GP induced an IgG response and provided protection from homologous challenge [58,59]. Phase 1b clinical studies for a MARV DNA plasmid vaccine (VRC-MARDNA025-00-VP) expressing MARV Angola DNA have been completed [51]. Although recombinant vesicular stomatitis virus (rVSV) vaccine incorporating MARV GP in place of its innate surface membrane GP appears promising for MARV, no Phase 1 clinical studies have been conducted to date. MARV virus-like particles (mVLP) vaccines have been developed using MARV VP40, GP and NP. In non-human primates, a mVLP vaccine against MARV-Musoke, Ravn, and Ci67 isolates was tested, and antibody responses to all three strains were observed [60].
Further reading
Articles (in alphabetical order)
Amman BR, Schuh AJ, Albarino CG, Towner JS. Marburg Virus Persistence on Fruit as a Plausible Route of Bat to Primate Filovirus Transmission. Viruses. 2021;13(12).
Cross RW, Mire CE, Feldmann H, Geisbert TW. Post-exposure treatments for Ebola and Marburg virus infections. Nat Rev Drug Discov. 2018;17(6):413-34.
Dulin N, Spanier A, Merino K, Hutter JN, Waterman PE, Lee C, et al. Systematic review of Marburg virus vaccine nonhuman primate studies and human clinical trials. Vaccine. 2021;39[1]:202-8.
Gordon TB, Hayward JA, Marsh GA, Baker ML, Tachedjian G. Host and Viral Proteins Modulating Ebola and Marburg Virus Egress. Viruses. 2019 Jan;11[1]:25.
Miraglia CM. Marburgviruses: An Update. Lab Med. 2019;50[1]:16-28.
Olejnik J, Hume AJ, Leung DW, Amarasinghe GK, Basler CF, Mühlberger E. Filovirus Strategies to Escape Antiviral Responses. Curr Top Microbiol Immunol. 2017;411:293-322.
Selvaraj SA, Lee KE, Harrell M, Ivanov I, Allegranzi B. Infection Rates and Risk Factors for Infection Among Health Workers During Ebola and Marburg Virus Outbreaks: A Systematic Review. J Infect Dis. 2018;218(suppl_5):S679-S689.
Suschak JJ, Pawęska JT, Storm N, Markotter W, Di Paola N, Wiley MR, Palacios G, Jansen van Vuren P. Shedding of Marburg Virus in Naturally Infected Egyptian Rousette Bats, South Africa, 2017. Emerg Infect Dis. 2020;26(12):3051-5.
Suschak JJ, Schmaljohn CS. Vaccines against Ebola virus and Marburg virus: recent advances and promising candidates. Hum Vaccin Immunother. 2019;15(10):2359-77.
Yu Z, Wu H, Huang Q, Zhong Z. Simultaneous detection of Marburg virus and Ebola virus with TaqMan-based multiplex real-time PCR method. J Clin Lab Anal. 2021;35(6):e23786.
Institutional resources (in alphabetical order)
Centers for Disease Control and Prevention. Marburg virus disease. Available at: https://www.cdc.gov/vhf/marburg
International Committee on Taxonomy of Viruses. (2020) Filoviridae. Available at: https://talk.ictvonline.org/ictv-reports/ictv_online_report/negative-sense-rna-viruses/w/filoviridae
World Health Organization. Marburg virus disease. Available at: https://www.who.int/news-room/fact-sheets/detail/marburg-virus-disease
World Health Organization Regional Office for Africa. Marburg Haemorrhagic Fever. Brazzaville: WHO Regional Office for Africa. Available at: http://afro.who.int/health-topics/marburg-haemorrhagic-fever
World Health Organization. (2014) Interim infection prevention and control guidance for care of patients with suspected or confirmed filovirus haemorrhagic fever in health-care settings, with focus on Ebola. Available at: https://apps.who.int/iris/handle/10665/130596
References
- European Centre for Disease Prevention and Control. Ebola and Marburg virus diseases - Annual Epidemiological Report for 2019. 2021.
- European Commission. Commission implementing decision 2018/945 of 22 June 2018 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. Luxembourg: Office of the European Union. 6.7.2018:L170/1. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945&from=EN#page=13.
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. . Luxembourg: Publications Office of the European Union. 22.12.2005:L 338. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:338:0001:0026:EN:PDF,
- ICTV. Genus Marburgvirus. Available at: https://ictv.global/report/chapter/filoviridae/filoviridae/marburgvirus
- Olejnik J, Muhlberger E, Hume AJ. Recent advances in marburgvirus research. F1000Research. 2019;8 Available at: https://www.ncbi.nlm.nih.gov/pubmed/31131088
- Gordon TB, Hayward JA, Marsh GA, Baker ML, Tachedjian G. Host and Viral Proteins Modulating Ebola and Marburg Virus Egress. Viruses. 2019 Jan 3;11(1) Available at: https://www.ncbi.nlm.nih.gov/pubmed/30609802
- Messaoudi I, Amarasinghe GK, Basler CF. Filovirus pathogenesis and immune evasion: insights from Ebola virus and Marburg virus. Nat Rev Microbiol. 2015 Nov;13(11):663-76. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26439085
- Valmas C, Grosch MN, Schumann M, Olejnik J, Martinez O, Best SM, et al. Marburg virus evades interferon responses by a mechanism distinct from ebola virus. PLoS Pathog. 2010 Jan 15;6(1):e1000721. Available at: https://www.ncbi.nlm.nih.gov/pubmed/20084112
- Valmas C, Basler CF. Marburg virus VP40 antagonizes interferon signaling in a species-specific manner. J Virol. 2011 May;85(9):4309-17. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21325424
- Centers for Disease Control and Prevention. Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition.
- Brauburger K, Hume AJ, Muhlberger E, Olejnik J. Forty-five years of Marburg virus research. Viruses. 2012 Oct 1;4(10):1878-927. Available at: https://www.ncbi.nlm.nih.gov/pubmed/23202446
- Asad A, Aamir A, Qureshi NE, Bhimani S, Jatoi NN, Batra S, et al. Past and current advances in Marburg virus disease: a review. Infez Med. 2020 Sep 1;28(3):332-45. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32920568
- Mehedi M, Groseth A, Feldmann H, Ebihara H. Clinical aspects of Marburg hemorrhagic fever. Future Virol. 2011 Sep;6(9):1091-106. Available at: https://www.ncbi.nlm.nih.gov/pubmed/22046196
- Shifflett K, Marzi A. Marburg virus pathogenesis - differences and similarities in humans and animal models. Virol J. 2019 Dec 30;16(1):165. Available at: https://www.ncbi.nlm.nih.gov/pubmed/31888676
- Ristanovic ES, Kokoskov NS, Crozier I, Kuhn JH, Gligic AS. A Forgotten Episode of Marburg Virus Disease: Belgrade, Yugoslavia, 1967. Microbiol Mol Biol Rev. 2020 May 20;84(2) Available at: https://www.ncbi.nlm.nih.gov/pubmed/32404328
- Zeller H. [Lessons from the Marburg virus epidemic in Durba, Democratic Republic of the Congo (1998-2000)]. Med Trop (Mars). 2000;60(2 Suppl):23-6. Available at: https://www.ncbi.nlm.nih.gov/pubmed/11100456
- Centers for Disease C, Prevention. Outbreak of Marburg virus hemorrhagic fever--Angola, October 1, 2004-March 29, 2005. MMWR Morb Mortal Wkly Rep. 2005 Apr 1;54(12):308-9. Available at: https://www.ncbi.nlm.nih.gov/pubmed/15800477
- Knust B, Schafer IJ, Wamala J, Nyakarahuka L, Okot C, Shoemaker T, et al. Multidistrict Outbreak of Marburg Virus Disease-Uganda, 2012. J Infect Dis. 2015 Oct 1;212 Suppl 2:S119-28. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26209681
- Nyakarahuka L, Shoemaker TR, Balinandi S, Chemos G, Kwesiga B, Mulei S, et al. Marburg virus disease outbreak in Kween District Uganda, 2017: Epidemiological and laboratory findings. PLoS Negl Trop Dis. 2019 Mar;13(3):e0007257. Available at: https://www.ncbi.nlm.nih.gov/pubmed/30883555
- Gear JS, Cassel GA, Gear AJ, Trappler B, Clausen L, Meyers AM, et al. Outbreake of Marburg virus disease in Johannesburg. Br Med J. 1975 Nov 29;4(5995):489-93. Available at: https://www.ncbi.nlm.nih.gov/pubmed/811315
- Johnson ED, Johnson BK, Silverstein D, Tukei P, Geisbert TW, Sanchez AN, et al. Characterization of a new Marburg virus isolated from a 1987 fatal case in Kenya. Arch Virol Suppl. 1996;11:101-14. Available at: https://www.ncbi.nlm.nih.gov/pubmed/8800792
- Smith DH, Johnson BK, Isaacson M, Swanapoel R, Johnson KM, Killey M, et al. Marburg-virus disease in Kenya. Lancet. 1982 Apr 10;1(8276):816-20. Available at: https://www.ncbi.nlm.nih.gov/pubmed/6122054
- Okonji OC, Okonji EF, Mohanan P, Babar MS, Saleem A, Khawaja UA, et al. Marburg virus disease outbreak amidst COVID-19 in the Republic of Guinea: A point of contention for the fragile health system? Clin Epidemiol Glob Health. 2022 Jan-Feb;13:100920. Available at: https://www.ncbi.nlm.nih.gov/pubmed/34901523
- Adjemian J, Farnon EC, Tschioko F, Wamala JF, Byaruhanga E, Bwire GS, et al. Outbreak of Marburg hemorrhagic fever among miners in Kamwenge and Ibanda Districts, Uganda, 2007. J Infect Dis. 2011 Nov;204 Suppl 3:S796-9. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21987753
- World Health Organisation Regional Office for Africa. Ghana declares first-ever outbreak of Marburg virus disease, 17 July 2022. Brazaville: WHO AFRO; 2022. Available at: https://www.afro.who.int/countries/ghana/news/ghana-declares-first-ever-outbreak-marburg-virus-disease-0
- Nikiforov VV, Turovskii Iu I, Kalinin PP, Akinfeeva LA, Katkova LR, Barmin VS, et al. [A case of a laboratory infection with Marburg fever]. Zh Mikrobiol Epidemiol Immunobiol. 1994 May-Jun(3):104-6. Available at: https://www.ncbi.nlm.nih.gov/pubmed/7941853
- Timen A, Koopmans MP, Vossen AC, van Doornum GJ, Gunther S, van den Berkmortel F, et al. Response to imported case of Marburg hemorrhagic fever, the Netherland. Emerg Infect Dis. 2009 Aug;15(8):1171-5. Available at: https://www.ncbi.nlm.nih.gov/pubmed/19751577
- Centers for Disease C, Prevention. Imported case of Marburg hemorrhagic fever - Colorado, 2008. MMWR Morb Mortal Wkly Rep. 2009 Dec 18;58(49):1377-81. Available at: https://www.ncbi.nlm.nih.gov/pubmed/20019654
- Schwartz DA. Maternal Filovirus Infection and Death from Marburg and Ravn Viruses: Highly Lethal to Pregnant Women and Their Fetuses Similar to Ebola Virus. IntechOpen; 2019, July 31. Available at: https://www.intechopen.com/chapters/68376
- World Health Organization. Marburg virus disease. 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/marburg-virus-disease
- Heeney JL. Ebola: Hidden reservoirs. Nature. 2015 Nov 26;527(7579):453-5. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26607539
- Coffin KM, Liu J, Warren TK, Blancett CD, Kuehl KA, Nichols DK, et al. Persistent Marburg Virus Infection in the Testes of Nonhuman Primate Survivors. Cell Host Microbe. 2018 Sep 12;24(3):405-16 e3. Available at: https://www.ncbi.nlm.nih.gov/pubmed/30173956
- Feldmann H. Virus in Semen and the Risk of Sexual Transmission. N Engl J Med. 2018 Apr 12;378(15):1440-1. Available at: https://www.ncbi.nlm.nih.gov/pubmed/29641967
- Slenczka W. Filovirus Research: How it Began. Curr Top Microbiol Immunol. 2017;411:3-21. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28766193
- Brainard J, Pond K, Hooper L, Edmunds K, Hunter P. Presence and Persistence of Ebola or Marburg Virus in Patients and Survivors: A Rapid Systematic Review. PLoS Negl Trop Dis. 2016 Feb;10(2):e0004475. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26927697
- Mupapa K, Mukundu W, Bwaka MA, Kipasa M, De Roo A, Kuvula K, et al. Ebola hemorrhagic fever and pregnancy. J Infect Dis. 1999 Feb;179 Suppl 1:S11-2. Available at: https://www.ncbi.nlm.nih.gov/pubmed/9988157
- Bebell LM, Riley LE. Ebola virus disease and Marburg disease in pregnancy: a review and management considerations for filovirus infection. Obstet Gynecol. 2015 Jun;125(6):1293-8. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26000499
- Jamieson DJ, Uyeki TM, Callaghan WM, Meaney-Delman D, Rasmussen SA. What obstetrician-gynecologists should know about Ebola: a perspective from the Centers for Disease Control and Prevention. Obstet Gynecol. 2014 Nov;124(5):1005-10. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25203368
- Borchert M, Muyembe-Tamfum JJ, Colebunders R, Libande M, Sabue M, Van Der Stuyft P. Short communication: a cluster of Marburg virus disease involving an infant. Trop Med Int Health. 2002 Oct;7(10):902-6. Available at: https://www.ncbi.nlm.nih.gov/pubmed/12358627
- Piercy TJ, Smither SJ, Steward JA, Eastaugh L, Lever MS. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol. 2010 Nov;109(5):1531-9. Available at: https://www.ncbi.nlm.nih.gov/pubmed/20553340
- Feldmann F, Shupert WL, Haddock E, Twardoski B, Feldmann H. Gamma Irradiation as an Effective Method for Inactivation of Emerging Viral Pathogens. Am J Trop Med Hyg. 2019 May;100(5):1275-7. Available at: https://www.ncbi.nlm.nih.gov/pubmed/30860018
- Jens Kuhn. Filoviruses: A Compendium of 40 Years of Epidemiological, Clinical, and Laboratory Studies. Austria: Springer-Verlad/Wien; 2008.
- Bauer MP, Timen A, Vossen A, van Dissel JT. Marburg haemorrhagic fever in returning travellers: an overview aimed at clinicians. Clin Microbiol Infect. 2019 Apr;21S:e28-e31. Available at: https://www.ncbi.nlm.nih.gov/pubmed/24816494
- Grolla A, Lucht A, Dick D, Strong JE, Feldmann H. Laboratory diagnosis of Ebola and Marburg hemorrhagic fever. Bull Soc Pathol Exot. 2005 Sep;98(3):205-9. Available at: https://www.ncbi.nlm.nih.gov/pubmed/16267962
- Saijo M, Niikura M, Ikegami T, Kurane I, Kurata T, Morikawa S. Laboratory diagnostic systems for Ebola and Marburg hemorrhagic fevers developed with recombinant proteins. Clin Vaccine Immunol. 2006 Apr;13(4):444-51. Available at: https://www.ncbi.nlm.nih.gov/pubmed/16603611
- Miraglia CM. Marburgviruses: An Update. Lab Med. 2019 Jan 1;50(1):16-28. Available at: https://www.ncbi.nlm.nih.gov/pubmed/30085179
- Emperador DM, Mazzola LT, Wonderly Trainor B, Chua A, Kelly-Cirino C. Diagnostics for filovirus detection: impact of recent outbreaks on the diagnostic landscape. BMJ Glob Health. 2019;4(Suppl 2):e001112. Available at: https://www.ncbi.nlm.nih.gov/pubmed/30899573
- Sherwood LJ, Osborn LE, Carrion R, Jr., Patterson JL, Hayhurst A. Rapid assembly of sensitive antigen-capture assays for Marburg virus, using in vitro selection of llama single-domain antibodies, at biosafety level 4. J Infect Dis. 2007 Nov 15;196 Suppl 2:S213-9. Available at: https://www.ncbi.nlm.nih.gov/pubmed/17940952
- Stonier SW, Herbert AS, Kuehne AI, Sobarzo A, Habibulin P, Dahan CVA, et al. Marburg virus survivor immune responses are Th1 skewed with limited neutralizing antibody responses. J Exp Med. 2017 Sep 4;214(9):2563-72. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28724616
- Rougeron V, Feldmann H, Grard G, Becker S, Leroy EM. Ebola and Marburg haemorrhagic fever. J Clin Virol. 2015 Mar;64:111-9. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25660265
- Kortepeter MG, Dierberg K, Shenoy ES, Cieslak TJ, Medical Countermeasures Working Group of the National Ebola T, Education Center's Special Pathogens Research N. Marburg virus disease: A summary for clinicians. Int J Infect Dis. 2020 Oct;99:233-42. Available at: https://www.ncbi.nlm.nih.gov/pubmed/32758690
- Raabea VN, Borcherta M. Infection control during filoviral hemorrhagic Fever outbreaks. J Glob Infect Dis. 2012 Jan;4(1):69-74. Available at: https://www.ncbi.nlm.nih.gov/pubmed/22529631
- World Health Organization. Interim advice on the sexual transmission of the Ebola virus disease. 2016. Available at: https://www.who.int/publications/m/item/interim-advice-on-the-sexual-transmission-of-the-ebola-virus-disease
- Finch CL, Martinez C, Leffel E, Skiadopoulos MH, Hacker A, Mwesigwa B, et al. Vaccine Licensure in the Absence of Human Efficacy Data. Vaccines (Basel). 2022 Feb 26;10(3) Available at: https://www.ncbi.nlm.nih.gov/pubmed/35335000
- Anywaine Z, Barry H, Anzala O, Mutua G, Sirima SB, Eholie S, et al. Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in children and adolescents in Africa: A randomised, placebo-controlled, multicentre Phase II clinical trial. PLoS Med. 2022 Jan;19(1):e1003865. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35015777
- Swenson DL, Wang D, Luo M, Warfield KL, Woraratanadharm J, Holman DH, et al. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin Vaccine Immunol. 2008 Mar;15(3):460-7. Available at: https://www.ncbi.nlm.nih.gov/pubmed/18216185
- Milligan ID, Gibani MM, Sewell R, Clutterbuck EA, Campbell D, Plested E, et al. Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial. JAMA. 2016 Apr 19;315(15):1610-23. Available at: https://www.ncbi.nlm.nih.gov/pubmed/27092831
- Falzarano D, Geisbert TW, Feldmann H. Progress in filovirus vaccine development: evaluating the potential for clinical use. Expert Rev Vaccines. 2011 Jan;10(1):63-77. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21162622
- Geisbert TW, Bailey M, Geisbert JB, Asiedu C, Roederer M, Grazia-Pau M, et al. Vector choice determines immunogenicity and potency of genetic vaccines against Angola Marburg virus in nonhuman primates. J Virol. 2010 Oct;84(19):10386-94. Available at: https://www.ncbi.nlm.nih.gov/pubmed/20660192
- Warfield KL, Aman MJ. Advances in virus-like particle vaccines for filoviruses. J Infect Dis. 2011 Nov;204 Suppl 3:S1053-9. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21987741




