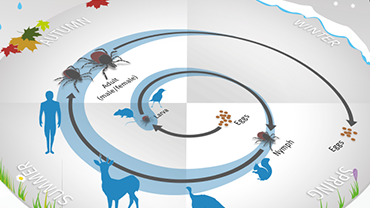
Tick-borne diseases
Ticks are abundant in woodlands all across Europe from early spring to late autumn. They live by sucking blood from animals and occasionally bite humans.
Ticks themselves do not cause disease but if a tick is infected with a virus or bacterium, then that pathogen can be transmitted through the tick’s bite and cause disease in humans.
Tick-borne encephalitis (TBE) is a common tick-borne disease in Europe (along with Lyme borreliosis).