Acute respiratory infections in the EU/EEA: epidemiological update and current public health recommendations – winter 2024/2025
Almost all countries reporting data to ECDC are observing sharp increases in indicators of both influenza and respiratory syncytial virus (RSV) activity, with a visible impact in secondary care.
Summary
Epidemiological context
- Almost all countries reporting data to ECDC are observing sharp increases in indicators of both influenza and respiratory syncytial virus (RSV) activity, with a visible impact in secondary care. The EU/EEA 10% primary care test positivity threshold signalling the start of the influenza season has been reached, with influenza A(H1N1)pdm09, A(H3N2) and B viruses co-circulating.
- Co-circulation of influenza viruses and RSV against the current background of continued relatively low-level transmission of SARS-CoV-2 could substantially impact healthcare services. Hospital admissions could occur in all age groups, with very young children (due to RSV) and older adults particularly affected. The impact of influenza may be worse if an A(H3N2) subclade that is less well matched with the northern hemisphere vaccine dominates.
- The end-of-year festive season is traditionally associated with activities such as social gatherings, shopping and travelling, which pose additional risks for intensified respiratory virus transmission.
Recommendations
- Member States should be prepared for continued increases in influenza and RSV activity during the coming weeks, and consider infection prevention and control practices to reduce transmission in healthcare settings, including long-term care facilities. They should also consider increasing primary and secondary healthcare system capacity.
- Immunisation is the most effective measure to protect against severe viral respiratory disease caused by certain viruses and scenario modelling has shown high vaccine uptake at the population level is strongly correlated with reduced disease burden.
- People eligible for vaccination against influenza, COVID-19 or RSV, particularly those at higher risk of severe outcomes, are encouraged to get vaccinated without delay in line with national recommendations to have the best chance of being protected.
- RSV immunoprophylaxis for infants, which have been shown to be safe and effective, can be considered per national guidelines.
- The updated COVID-19 vaccines offered as a booster for winter 2024-2025 should provide improved protection against severe outcomes from the currently circulating SARS-CoV-2 variants compared to the XBB.1.5-targeting COVID-19 vaccine booster offered in autumn 2023.
- Clinicians should be reminded that, when indicated in national guidelines, the early use of antiviral treatments for influenza and COVID-19 may prevent progression to severe disease in vulnerable groups.
- For influenza, it may be prudent to have a lower threshold for antiviral use in risk groups, if an influenza virus subclade not well matched with the northern hemisphere vaccine dominates.
- SARS-CoV-2 monoclonal antibodies currently authorised in the EU/EEA lack efficacy against variants carrying the F456L mutation, which is widely present in currently circulating XEC, KP.3 and BA.2.86 lineages. However, available antiviral treatments are expected to remain effective.
- Unusual events or clusters of respiratory infections, for example adenovirus or Mycoplasma pneumoniae, not monitored by routine surveillance but placing additional strain on healthcare systems, should be reported through EpiPulse. Additionally, clusters of atypical or particularly severe presentations for influenza (or suspected zoonotic avian influenza), RSV and SARS-CoV-2 should also be reported.
Epidemiological trends in the EU/EEA
Respiratory virus activity
A summary of the latest respiratory virus epidemiological situation in the EU/EEA, focusing on influenza, RSV and SARS-CoV-2 is published weekly by ECDC in the European Respiratory Virus Surveillance Summary (ERVISS).
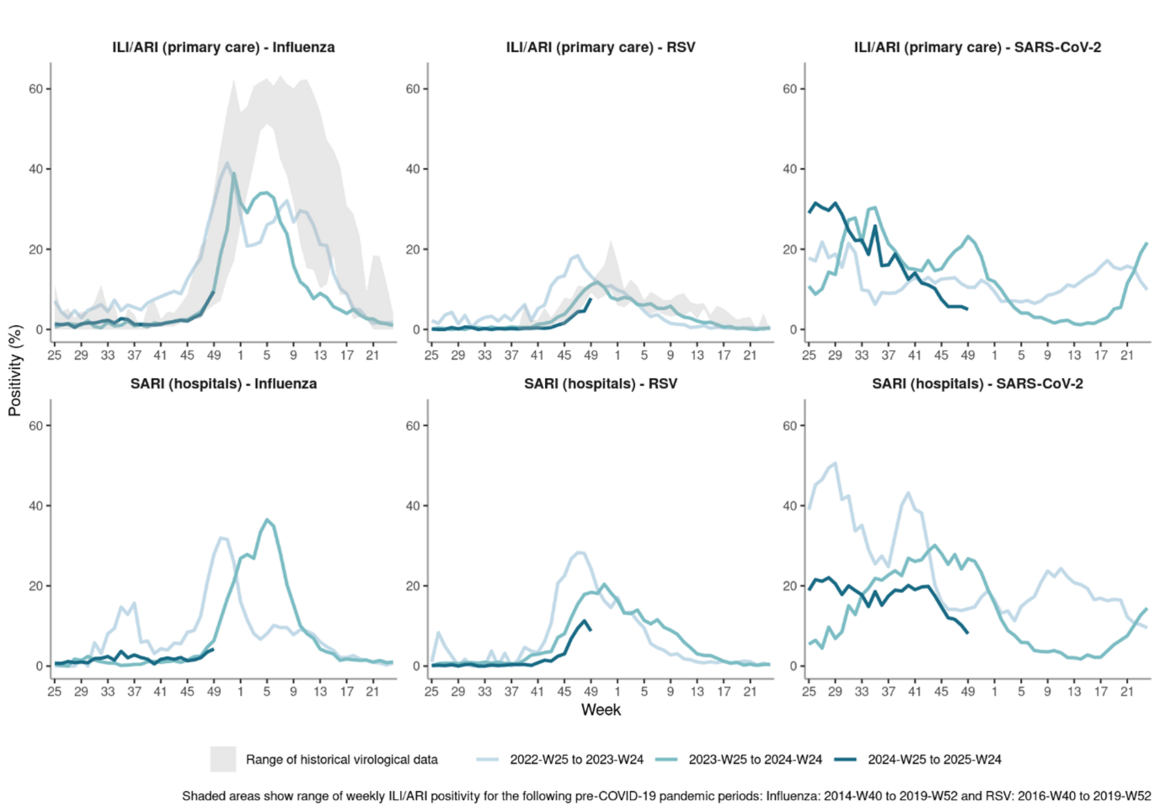
As of week 49, 2024, sharp rises over the past few weeks in indicators of virus activity (test positivity from ILI/ARI surveillance in primary care and/or detections or test positivity from non-sentinel laboratory-based surveillance) point to rapidly increasing community circulation of influenza viruses and RSV, affecting most of the countries reporting data. At the EU/EEA level, the influenza primary care test positivity threshold of 10% for the start of the influenza season was reached.
Trends from the last two years suggest a return to a more regular timing of seasonal influenza and RSV epidemics following the COVID-19 pandemic. The trend in influenza positivity is very similar to last year in terms of timing and rate of increase, while RSV is increasing with a roughly two-week delay compared to last year. SARS-CoV-2 activity has stabilised at relatively low levels in all countries following a prolonged period of elevated activity over the summer and autumn. This, alongside a lack of predictable seasonal pattern for SARS-CoV-2, makes it unclear whether we will experience a new SARS-CoV-2 epidemic this winter (Figure 1).
Figure 1. Proportion of positive tests for influenza, RSV and SARS-CoV-2 among swabbed patients presenting with ILI/ARI to primary care or SARI to hospitals, aggregated data from EU/EEA countries
Severe disease
SARI surveillance data to week 49, 2024 in ERVISS showed increasing trends in the number and proportion of hospital admissions testing positive for both RSV and influenza. Unlike influenza and RSV, which see activity confined to clear seasonal epidemics, a continuous background of severe COVID-19 has been previously observed, even when activity is at low levels. Accordingly, the number of patients admitted to hospital who tested positive for SARS-CoV-2 remained at similar levels to those observed for RSV.
While hospital admissions due to influenza have been observed in all age groups to date, individuals aged 65 years and older tend to have the highest risk of hospitalisation and severe outcomes. The age groups most affected by severe influenza this season may be influenced by which subtype(s) dominate.
Children aged 0-4 years, and in particular young infants, had the highest risk of hospitalisation for RSV (with over 40% of tests among SARI admissions in this age group positive for RSV since week 47, 2024). This age group, followed by those aged 65 years and above, accounted for 83% and 11%, respectively, of the RSV-positive SARI admissions between weeks 40-49, 2024. Subsequent intensive care unit (ICU) admissions occur most commonly in infants, while reported deaths tend to be concentrated in older age groups.
Among those infected by SARS-CoV-2, individuals aged 65 years and older remain the age group at highest risk of hospitalisation and severe COVID-19 outcomes, with 84% of individuals hospitalised with COVID-19 between weeks 40-49, 2024 in this age group.
To date, pooled excess mortality reported by EuroMOMO remained within the normal range.
Virological surveillance, implications for immunisation and treatment
Influenza
Data in ERVISS show that all three influenza virus types/subtypes (A(H1N1)pdm09, A(H3N2) and B) are currently co-circulating, accounting for around two-fifths, one-fifth and two-fifths, respectively, of detections among the B (B/Victoria or B (unknown)) and subtyped A viruses reported from ILI/ARI virological surveillance between weeks 40-49, 2024. The overall type/subtype distribution in the season to date, with a high proportion of B viruses, differs from the past two seasons (an initial wave dominated by A(H3N2) before replacement by influenza B in 2022/2023, while A(H1N1)pdm09 was the dominant subtype across the 2023/2024 season) (Figure 2). This picture may be driven largely by Spanish data; Spain has a large primary care surveillance system and was one of only three countries with B dominance among the 20 countries reporting sufficient data to weeks 48 or 49, 2024 for the calculation of weekly dominant type.
At current levels of influenza circulation, it is too early to predict how the distribution of circulating influenza types and subtypes will evolve, nor which strains or clades will dominate later this winter. By way of comparison, the influenza season in Australia was co-dominated by A(H1N1)pdm09 (47.9%) and A(H3N2) (47.7%) viruses and with very little circulation of B/Victoria-lineage viruses (4.4%).
Figure 2. Weekly and cumulative distributions of circulating influenza types and subtypes, seasons 2022/23 to 2024/25, aggregated data from EU/EEA countries
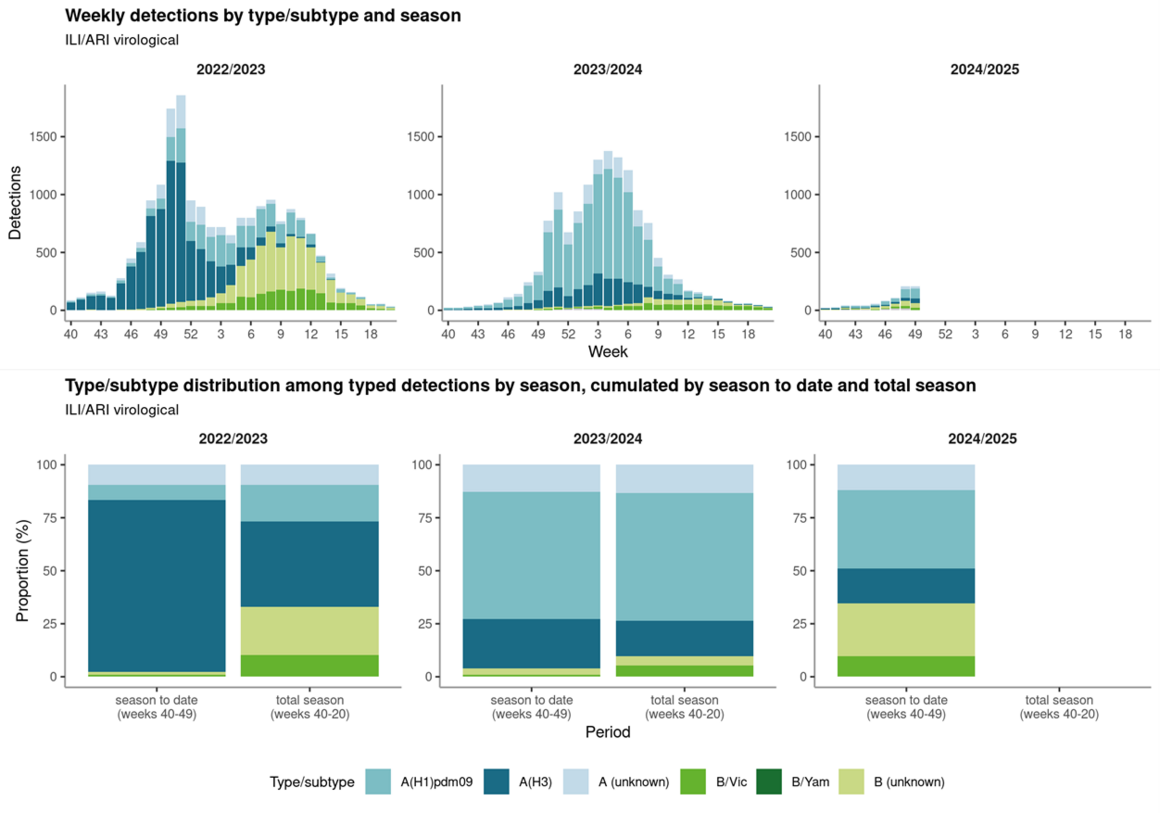
Virus characterisation data reported by EU/EEA countries since week 40, 2024 is summarised in ERVISS, but does not give full details on the circulating strains in the EU/EEA since it is still early in the influenza season.
Current northern hemisphere vaccine components are well matched to circulating 5a.2a and 5a.2a.1 A(H1N1)pdm09 and V1A.3a.2 B/Victoria subclades. The vaccine components appear well matched for the A(H3N2) 2a.3a.1 (J) clade viruses, but less well matched for some of the more recent A(H3N2) subclade 2a.3a.1 (J2) viruses, characterised by S145N, N158K or K189R HA substitutions (alone or in combination). The majority of the A(H3N2) viruses identified worldwide and in Europe since February 2024 belong to the subclade 2a.3a.1 (J2).
The majority of currently circulating influenza strains are susceptible to commonly used antiviral drugs against influenza, with very few detected viruses displayed in ERVISS having shown reduced susceptibility against neuraminidase inhibitors or baloxavir marboxil this far this season.
RSV
RSV is not regularly typed in the laboratories and the type has not been shown to affect the vaccine or monoclonal antibody effectiveness yet. However, ERVISS data on circulating RSV types from ILI/ARI virological surveillance, submitted by seven countries, show an equal proportion of RSV-A and RSV-B.
Several RSV immunisation products have been authorised in the EU over the last two years. These include active immunisation products to protect older adults through vaccination (including the vaccines Arexvy, Abrysvo and mResvia) and passive immunisation products to protect newborns and infants through maternal vaccination (Abrysvo) or single-dose long-acting monoclonal antibody (Beyfortus). Pre-authorisation clinical trials and data from post authorisation studies collected from the 2023-2024 RSV season have shown that these products are safe and provide protection against RSV-associated hospitalisation and severe disease in infants and older adults. Countries in the EU/EEA are at different stages of issuing recommendations for their use and product rollout.
SARS-CoV-2
ECDC uses three classification categories to communicate increasing levels of concern about new or emerging SARS-CoV-2 variants: variant under monitoring (VUM), variant of interest (VOI) and variant of concern (VOC). There are currently no SARS-CoV-2 variants meeting the VOC classification criteria. BA.2.86 and its descendent lineages—including KP.3—are classified as VOIs. KP.3 was dominant at the most recent peak of SARS-CoV-2 activity in July 2024. Since then, the proportion of SARS-CoV-2 variants belonging to KP.3 has declined in the EU/EEA, with XEC—a recombinant of two BA.2.86 descendants: KS.1.1 and KP.3.3—gradually emerging as the dominant SARS-CoV-2 variant in circulation. XEC was classified as a VUM in September 2024. As of 7 December 2024, for the period weeks 47-48, 2024, variants are circulating at the following median proportions in the EU/EEA: XEC (46%), KP.3 (36%), BA.2.86 (17%). These estimates are based on data reported by eight countries submitting at least ten sequences to GISAD EpiCoV in the period.
There is currently no evidence of increased clinical severity for XEC relative to previously dominant SARS-CoV-2 variants and no evidence of increased COVID-19 hospitalisations, ICU admissions or deaths overall in those EU/EEA countries where XEC dominates. Available in vitro studies evaluating the ability of serum from previously infected and/or vaccinated individuals to neutralise XEC pseudoviruses indicates that an increased ability to evade neutralising antibodies has contributed to the growth advantage observed for XEC [links to studies: 1, 2, 3, 4, 5]. Specifically, a study in Norway evaluated serum collected in April/May 2024 from 97 seniors aged 68-82 years and demonstrated that neutralising responses were lower against XEC than for previously circulating BA.2.86 descendants. Responses were also lower amongst those who received the XBB.1.5-targeting COVID-19 vaccine booster in autumn 2023. Whilst real-world clinical studies of vaccine effectiveness are still awaited, in vitro studies have shown that serum from individuals who received the updated JN.1-targeting COVID-19 vaccine booster demonstrated significantly improved neutralisation of XEC [links to studies: 1, 2], in support of the current recommendation of an updated COVID-19 vaccine booster to provide improved protection against severe outcomes in this age group during winter 2024-2025.
Conclusions and recommendations for public health actions
Immunisation and antivirals
Influenza and SARS-CoV-2
ECDC recently published results from its inaugural RespiCompass modelling initiative. Scenario modelling was used to provide mid- to long-term projections of influenza burden (using primary care consultation data) and COVID-19 burden (using hospital admission data) for individuals aged 65 years and older for winter and spring 2024-2025. Possible vaccine coverage scenarios were explored for winter 2024-2025, leveraging EU/EEA country-specific influenza and COVID-19 vaccine coverage data. There was a strong correlation between vaccine coverage and decreased burden for both influenza and COVID-19 in winter/spring 2024-2025 across the EU/EEA. In countries with a higher absolute vaccine coverage, the relative reduction in burden was projected to be higher.
Vaccination against influenza and COVID-19 remains the single most effective measure for preventing infection and the development of severe disease among the elderly and persons with underlying medical conditions. High-risk groups for severe disease include the elderly, children aged six months to four years, pregnant women regardless of trimester, immunosuppressed individuals or people with chronic medical conditions. Influenza and COVID-19 vaccination campaigns are underway in the EU/EEA. Several countries are running combined vaccination campaigns that include both COVID-19 and influenza. Data for vaccine uptake will be available later in the season.
People eligible for vaccination against COVID-19 or influenza, who are at higher risk of severe disease and who have not yet been vaccinated are strongly encouraged to do so as soon as possible to have the best chance of being protected when virus circulation is at its highest in the coming weeks.
Healthcare workers should also be encouraged to get vaccinated against influenza to reduce the risk of infecting vulnerable groups and to protect themselves.
Clinicians should be reminded that, when indicated, the early use of antivirals against influenza may prevent progression to severe disease in vulnerable groups. Should influenza A(H3N2) become the dominating influenza subtype, it may be prudent to have a lower threshold for antiviral use in risk groups due to the potential for lower protection offered by vaccination against some clades of the virus.
Clinicians are advised to consider the latest available evidence of efficacy for SARS-CoV-2 monoclonal antibodies against predominantly circulating variants and whether administration will offer any clinical benefit. The European Medicines Agency recently published an update on the loss of activity of anti-spike protein monoclonal antibodies for SARS-CoV-2 variants carrying the F456L mutation, which is widely present in currently circulating XEC, KP.3 and BA.2.86 variants. However, antiviral drugs such as nirmatrelvir/ritonavir (Paxlovid) and remdesivir (Veklury) are available and approved in the EU for COVID-19 patients at high risk of severe illness and are expected to retain antiviral activity against currently circulating variants as their efficacy has not been affected by the mutations seen recently.
RSV
Immunisation against RSV is particularly important when RSV is circulating. RSV vaccines and monoclonal antibodies protect against RSV hospitalisation. Pregnant mothers and the elderly are encouraged to check whether they or their newborns are eligible for immunisation against RSV according to the recommendations currently in place in their country.
Infection, prevention and control and clinical management
The end-of-year festive season is traditionally associated with activities such as social gatherings, shopping and travelling, which pose additional risks for intensified respiratory virus transmission.
Member States should prepare for the possible need to increase emergency department and ICU capacity (in terms of adequate staffing and bed capacity), for both adult and paediatric hospitals. Hospital administrators and managers should ensure that resources, such as medical/nursing staff and equipment are also available.
Ensuring healthcare staff implement appropriate infection prevention and control (IPC) measures will reduce the burden and likelihood of outbreaks in healthcare settings, including in long-term care facilities. Clinicians should be reminded that, when indicated, the early use of antiviral treatments for COVID-19 and influenza may prevent progression to severe disease in vulnerable groups.
Risk communication activities for the public should be implemented, including targeted guidance for risk groups, healthcare workers and caretakers of vulnerable groups. Key recommendations include vaccination according to national recommendations, staying home when ill, respiratory etiquette and good hand hygiene, appropriate ventilation of indoor spaces, and promotion of appropriate public health and social measures (PHSM) (also called non-pharmaceutical interventions (NPIs)). People with high risk for severe disease (as well as their caretakers and close contacts) should consider using a face mask when in crowded public spaces.
Surveillance and reporting
Guidance on surveillance of respiratory viruses in the EU/EEA is provided in Operational Considerations for respiratory virus surveillance in Europe. Specimens from ILI/ARI/SARI patients should be tested using multiplex tests for respiratory viruses including influenza A and B, RSV and SARS-CoV-2 and data reported weekly to The European Surveillance System (TESSy).
ECDC assesses the risk of infection with currently circulating avian A(H5) influenza viruses in Europe as low for the general public in the EU/EEA and low-to-moderate for those occupationally or otherwise exposed to infected animals or contaminated environments. It is important to maintain awareness and continue to perform adequate surveillance to detect potential cases of zoonotic influenza during the influenza season. Ideally, all influenza-positive specimens from both ILI/ARI/SARI surveillance in primary and secondary care should be typed and subtyped. Specimens that are positive for influenza type A, but negative in subtyping assays of seasonal influenza viruses, should be sent to the national influenza reference laboratory, sub-typed for zoonotic influenza (including A(H5)) and, if zoonotic influenza is confirmed, characterised (including sequencing). If there are zoonotic influenza outbreaks in animals in the area, even in the absence of known exposure to infected animals, laboratories/clinicians are encouraged to increase testing and (sub)typing of influenza-A positive cases.
Recommendations for surveillance and targeted testing for the early detection of zoonotic influenza in humans during 2024-2025 winter period are provided in Surveillance and targeted testing for the early detection of zoonotic influenza in humans during the winter period in the EU/EEA.
Unusual events or clusters of respiratory infections, for example adenovirus or Mycoplasma pneumoniae, not monitored by routine surveillance but placing additional strain on healthcare systems, should be reported through EpiPulse. Additionally, clusters of atypical or particularly severe presentations for influenza (or suspected zoonotic avian influenza), RSV and SARS-CoV-2 should also be reported.
Consulted ECDC experts
Eeva Broberg, Nick Bundle, Kate Olsson, Ajibola Omokanye.