Weekly influenza update, week 8, February 2018
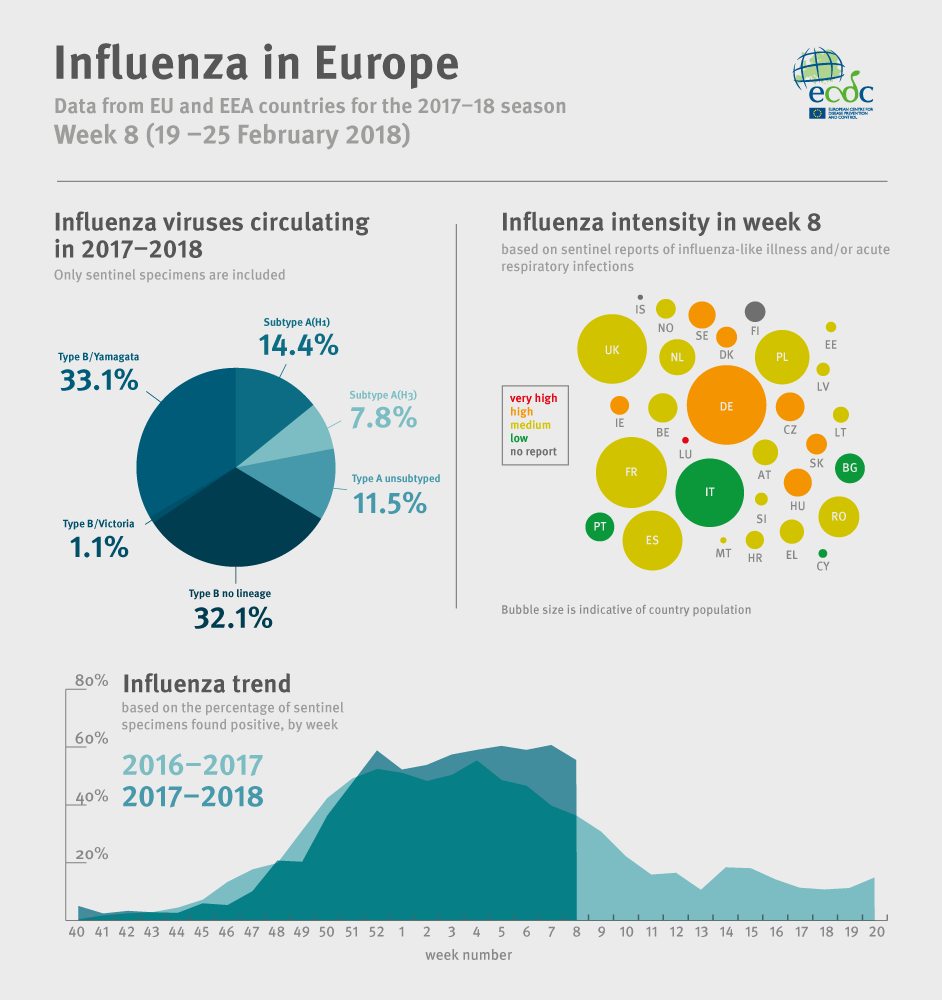
Influenza activity was widespread in the majority of reporting countries. Overall, 49% of individuals sampled from primary healthcare settings tested positive for influenza virus, a slight decrease compared to the previous week (51%). Both influenza virus types A and B were co-circulating with a higher proportion of type B viruses. Differences in proportions of circulating influenza virus types and A subtypes were observed between countries. The majority of severe cases admitted to non-ICU hospital wards were adults infected by influenza type B viruses. Half of severe cases admitted to ICU were adults infected by influenza type A viruses. Based on data provided by 20 EU countries to EuroMOMO, excess mortality from all causes has elevated significantly over the past months in the south-western part of the European region, but this increase seems less in some countries.
Download

2017/18 season overview
- For the region overall, a higher proportion of type B compared to type A viruses has been detected in sentinel and non-sentinel sources, representing a high level of circulation of influenza B viruses compared with previous seasons. Of the type A virus detections from sentinel sources, the majority of which were subtyped, A(H1N1)pdm09 viruses have outnumbered A(H3N2) viruses. In non-sentinel sources, more A(H3N2) viruses were reported than A(H1N1)pdm09 viruses.
- The majority of severe cases reported this season are due to influenza B and occur in persons above the age of 15 years. In laboratory-confirmed influenza cases in ICU, comparable numbers were infected by influenza type A and B viruses, and the elderly were at increased risk of ICU admission. In laboratory-confirmed influenza cases reported from wards other than ICU, type B viruses were detected approximately twice as frequently as type A viruses.
- For type B viruses from both sentinel and non-sentinel sources, B/Yamagata lineage viruses have greatly outnumbered those of the B/Victoria lineage. The current trivalent seasonal influenza vaccine does not include a virus from the B/Yamagata lineage.
- Different patterns of dominant type and A subtypes were observed between the countries of the Region, which may be due to differences in relative weights of information being derived from sentinel, non-sentinel and severe influenza case sources of information.
- Different patterns of dominant type and A subtype were observed between the countries of the Region, which may be due to differences in relative weights of information being derived from sentinel, non-sentinel and severe influenza case sources of information.
- While low in number, 59% of the genetically characterized A(H3N2) viruses belong to clade 3C.2a, the clade of the vaccine virus described in the WHO recommendations for vaccine composition for the northern hemisphere 2017–2018, and 37% to subclade 3C.2a1, with mammalian cell-cultured viruses in both clades being antigenically similar.
- Although few B/Victoria lineage viruses have been detected, their characterization has shown an increasing percentage (currently 47%) of viruses belonging to a subclade of clade 1A viruses, represented by B/Norway/2409/2017. These viruses have a two amino acid deletion in haemagglutinin (Δ162-163) and are antigenically different from the current trivalent vaccine component, a B/Brisbane/60/2008-like virus.
- Interim or real-time vaccine effectiveness estimates from Canada, Finland, Germany, Spain, Stockholm County and the United States of America suggest overall vaccine effectiveness of 15–46%, depending on the proportions of circulating (sub)types. Effectiveness against influenza B is in the range of 16–67%, despite the circulating lineage not being included in the most commonly used trivalent vaccine.
- Additional information on global influenza activity is available from WHO’s biweekly global updates.
- 19–21 February 2018, WHO-HQ convened the Vaccine Composition Meeting, which made recommendations for the composition of the 2018–2019 northern hemisphere vaccine.







