Technical guidelines on the prevention of donor-derived transmission of communicable diseases through Substances of Human Origin
On 17 July 2024, the new Regulation (EU) 2024/1938 on standards of quality and safety for substances of human origin intended for human application was published. After entry into force, in August 2027 for most provisions, the regulation will repeal the Blood Directive (2002/98/EC) and the Tissues and cells Directive (2004/23/EC). This regulation covers blood, tissues, cells, medically assisted reproduction, and other substances of human origin for human applications, with the exception of human organs for transplantation.
The SoHO regulation establishes ECDC as the expert body for developing and updating technical guidelines on the safety and quality of SoHOs from a communicable disease threat perspective. The proposed Regulation stipulates that SoHO entities in the Member States shall take into account “the most recent technical guidelines, as indicated on the EU SoHO Platform […] published by the ECDC concerning the prevention of communicable disease transmission” (article 56(4) and Article 59(4)).
In this context, ECDC is developing technical guidelines on the prevention of donor-derived communicable disease transmission through SoHO in the European Union and European Economic Area (EU/EEA).
Development of the technical guidelines
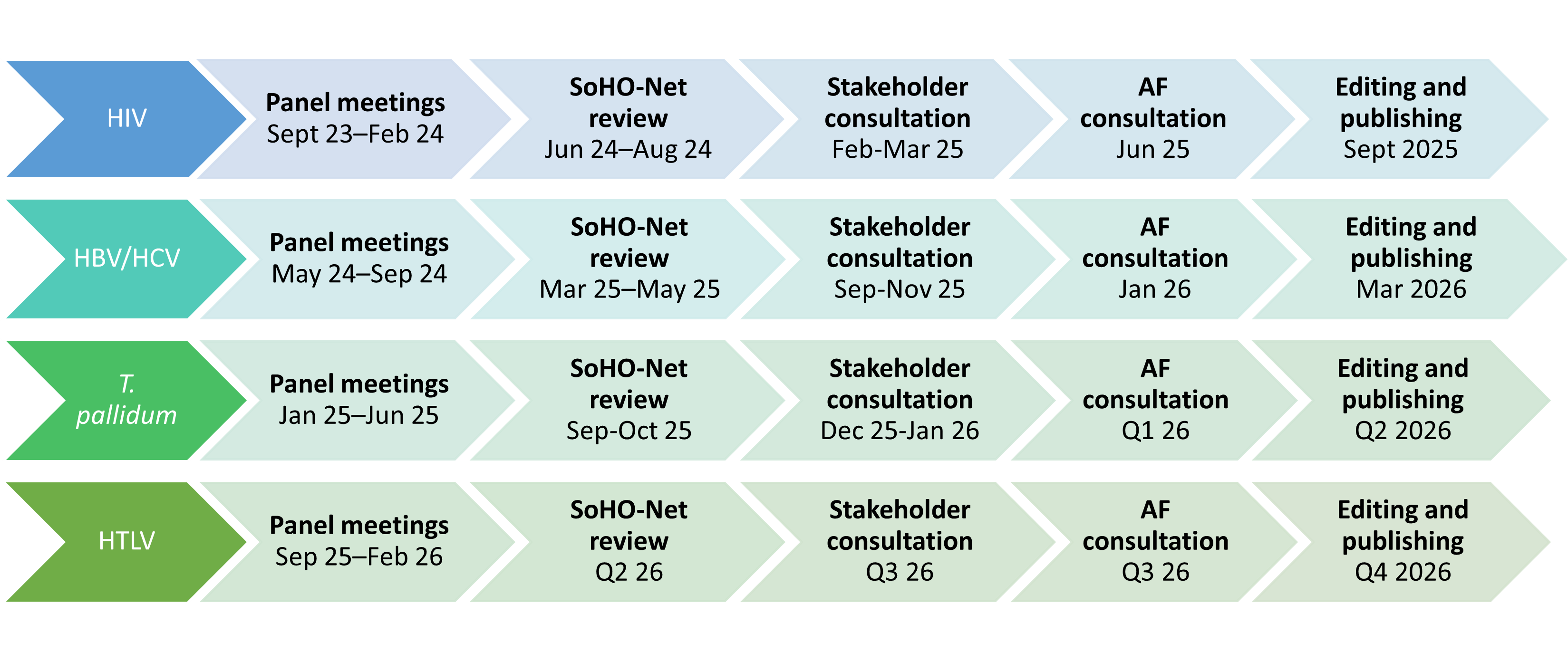
In June 2023, ECDC convened a scientific expert panel which included experts from the different SoHO areas (blood, tissues, cells, and medically assisted reproduction) and infectious diseases originating from 13 EU/EEA countries. This panel will support ECDC in developing guidelines for the prevention of transmission of human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), T. pallidum, and West Nile virus (WNV). In the first quarter of 2025, ECDC, in agreement with the expert panel, decided to replace WNV by the Human T-lymphotropic virus (HTLV) for the work of the panel.
Further scientific expert panels will be convened in the future to address additional pathogens, highlighted as a priority by the ECDC SoHO network. The upcoming batch of pathogens for which ECDC will initiate work in 2026 will cover relevant arboviruses, including: WNV, dengue virus, chikungunya virus, Zika virus, tick-borne encephalitis virus, and Crimean-Congo hemorrhagic fever virus.
The European Directorate for the Quality of Medicines and Healthcare (EDQM) of the Council of Europe is represented by two observers in the scientific expert panel to avoid inconsistencies and gaps between the guidelines developed by ECDC and those developed by EDQM.
ECDC provides the scientific evidence available in the form of evidence syntheses to support the expert panel discussions on relevant measures to prevent the transmission of pathogens through SoHO during the meetings of the panel. Outputs from the expert panel meetings will support ECDC in the development of technical guidelines on the prevention of donor-derived transmission of communicable diseases through SoHO.
The technical guidelines developed by ECDC will cover the testing and deferral strategies and laboratory testing methods for potential SoHO donors. According to the definition of SoHO in the regulation, these guidelines will not cover organ donors. Additionally, in their first iteration, these guidelines will not cover breast milk, faecal microbiota, or plasma collected for fractionation.
Review and approval of the technical guidelines
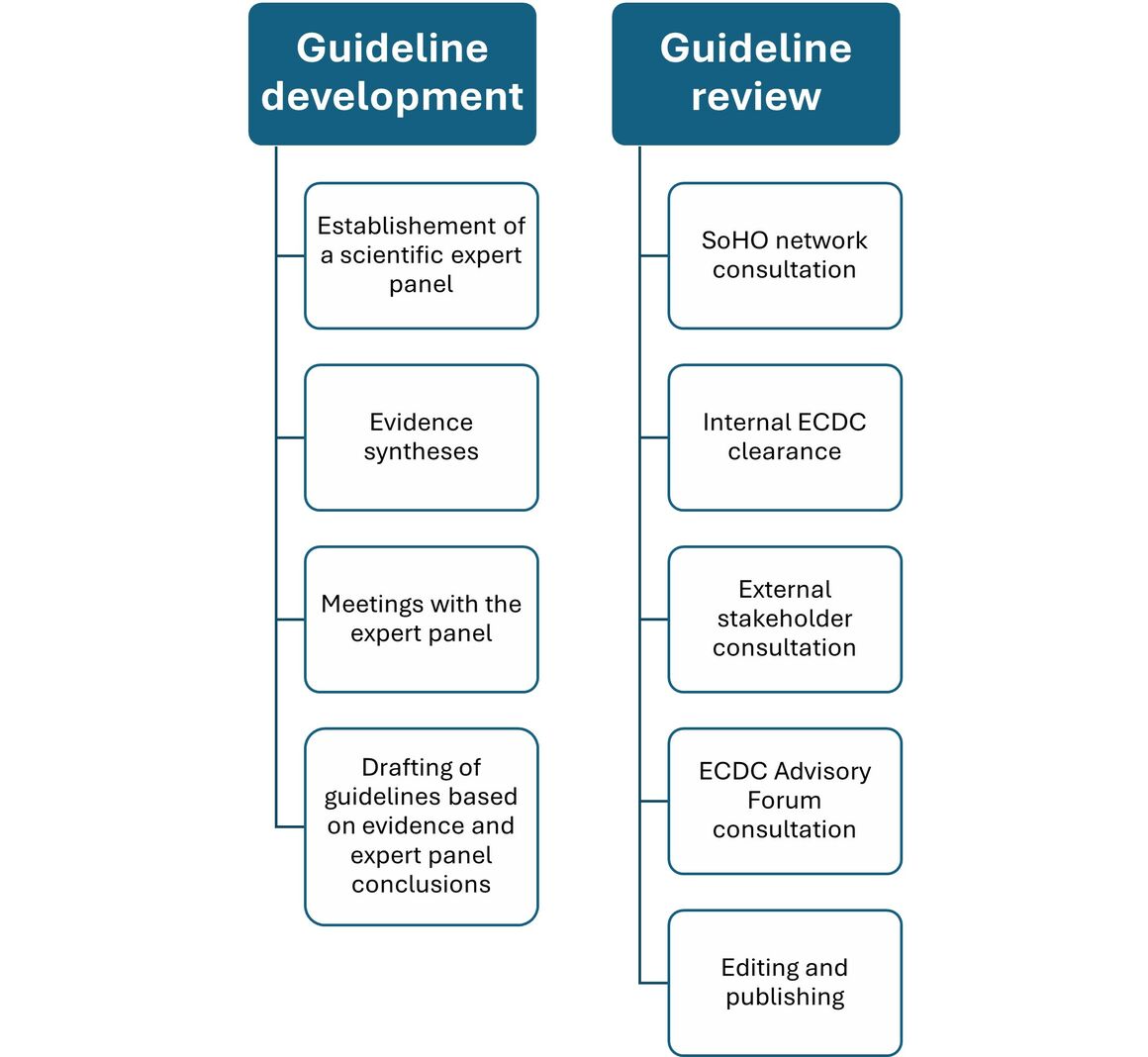
The ECDC SoHO network is consulted for the review of the draft technical guideline for a pathogen in the first step.
Following the ECDC SoHO network review and the internal ECDC scientific clearance, the technical guideline is sent for an external stakeholder consultation based on the list of stakeholder organisations established by the European Commission as well as to the EDQM, the European Medicines Agency (EMA), and the World Health Organization, Regional Office for Europe (WHO/Europe), the SoHO Coordination Board, and the ECDC Advisory Forum. Organisations interested in reviewing the guidelines can register on the list of stakeholder organisations established by the European Commission.
The final guidelines will be published on the ECDC website and the EU SoHO platform described in the SoHO regulation.
Timelines for the development of guidelines